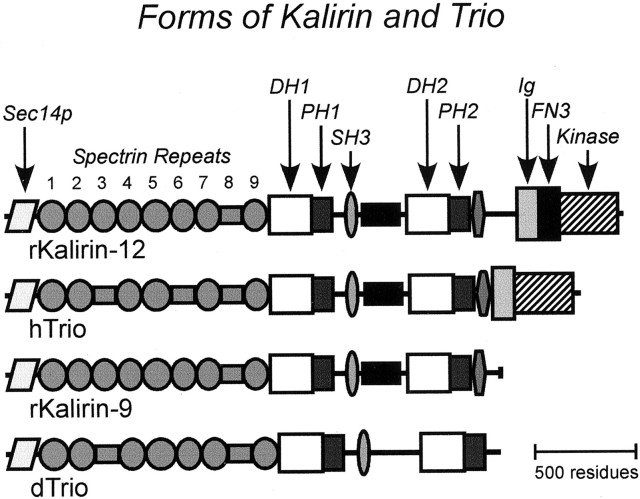
Fig. 1.
Kalirin and Trio. The structures of two isoforms of rat Kalirin (rKalirin-12; accession number AF232669;rKalirin-9; accession number AF232668), human Trio (hTrio; accession number AAC34245), and dTrio (accession number AB035419) are drawn to scale. Kalirin and Trio possess two distinct Dbl-homology (DH)/pleckstrin-homology (PH) domains typical of GEFs for Rho GTPases along with a putative serine/threonine kinase domain. In addition, both hTrio and rKalirin possess a Sec14p domain, multiple spectrin-like repeats, SH3 domains, and Ig/fibronectin III (FN3) domains. Among the Kalirin isoforms that arise from alternative splicing, Kalirin-12 protein is structurally similar to hTrio. Kalirin-9, like dTrio, is devoid of the kinase domain; Kalirin-7 lacks the second GEF domain, and GEF1 is followed instead by a C-terminal postsynaptic density-95/discs large/zona occludens-1-binding motif mediating Kalirin-7 enrichment in postsynaptic density fractions (Penzes et al., 2000, 2001).