Fig. 3.
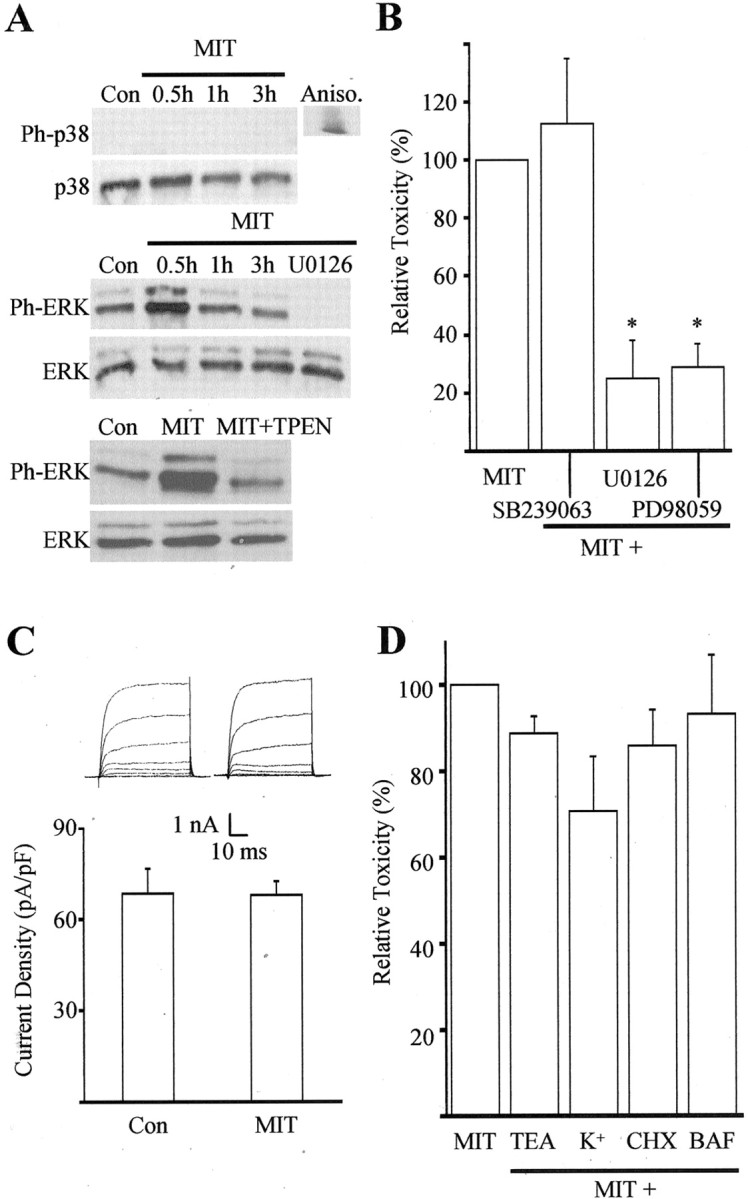
MIT toxicity involves ERK activation.A, Immunoblots of cell extracts from cortical cultures harvested at various time points after a 10 min exposure to 100 μm MIT. Proteins were separated by SDS-PAGE and probed with antibodies specific to the phosphorylated and nonphosphorylated forms of both p38 and p44/42 ERK. Note the absence of phosphorylated p38 (Ph-p38) at all time points and the early increase in phosphorylated ERK (Ph-ERK) after MIT exposure. The MEK inhibitor U0126 (10 μm) and the zinc chelator TPEN (1 μm) completely blocked ERK phosphorylation (30 min). Similar results were obtained in three additional experiments. Aniso, Anisomycin;Con, control. B, MIT toxicity was not blocked by the p38 inhibitor SB239063 (20 μm) but was significantly inhibited by the MEK inhibitors U0126 (10 μm) and PD98059 (40 μm). Results represent the mean ± SEM of three to four independent experiments; ∗p < 0.05. C, A lack of p38 involvement in MIT toxicity was confirmed by a lack of enhancement in potassium channel currents 3–4 hr after a 10 min MIT (100 μm) exposure (compare with McLaughlin et al., 2001). Results represent the mean ± SD current density (n = 6, 11) for potassium currents evoked in voltage-clamped cortical neurons by stepping the voltage to −10 mV from a holding voltage of −70 mV. Con, Control.Insets, Examples of whole-cell potassium currents obtained in two separate cortical neurons ∼3 hr after a 10 min exposure to vehicle or MIT. Currents were evoked by a series of steps to 35 mV from a holding voltage of −70 mV. D, Lack of neuroprotection against MIT by the following agents: TEA (10 mm), high extracellular potassium (25 mm), cyclohexamide (CHX, 3.5 μm), and BAF (20 μm); data represent the mean ± SEM (n = 4–9).
