Fig. 8.
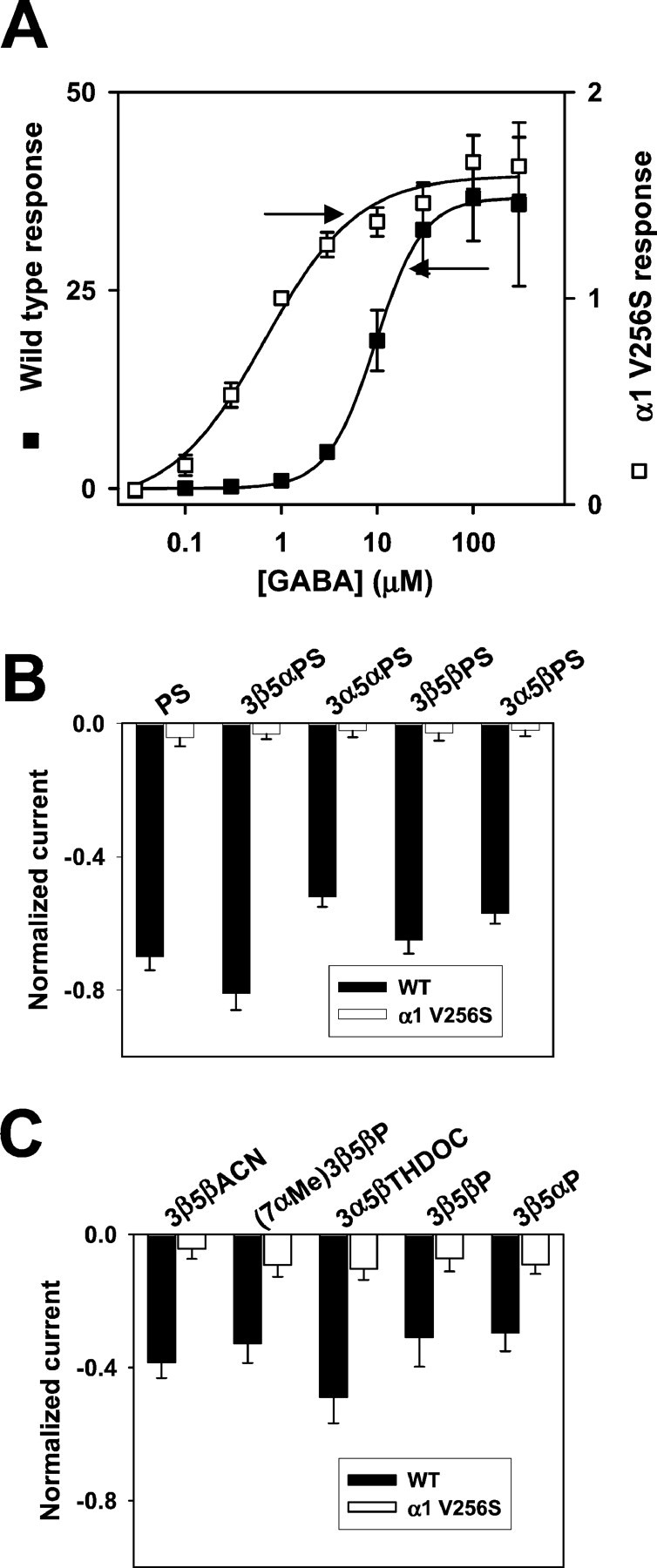
A point mutation in the α1 subunit (V256S) reduces sulfate and 3β-hydroxysteroid block of GABAAreceptors. A, GABA concentration–response curves for wild-type (filled squares; n= 5) and mutated (open squares; n = 6) GABAA receptors. Responses at 1 μm were set to 1 for both wild-type and mutated receptors, and other responses in the same oocyte were normalized to this response. Note that thelefty-axis corresponds to the wild-type normalized responses, and the righty-axis corresponds to the normalized responses from mutated receptors. Absolute amplitudes of maximum responses did not notably differ between wild-type and mutated receptors, but the mutation resulted in an ∼20-fold leftward shift in the GABA EC50, from 9.6 to 0.53 μm, resulting in the apparently larger normalized maximum responses for wild-type receptors. Arrows indicate concentrations of GABA used in subsequent studies of steroid block of GABAA receptors (B, C). B, Sulfated steroid effects (1 μm) in wild-type (WT,filled bars; n = 5) and α1V256S mutated (open bars; n = 5) GABAA receptors. C, 3β-Hydroxysteroid effects (10 μm) in wild-type (WT,filled bars; n = 6) and α1V256S (open bars; n = 9) mutated GABAA receptors.
