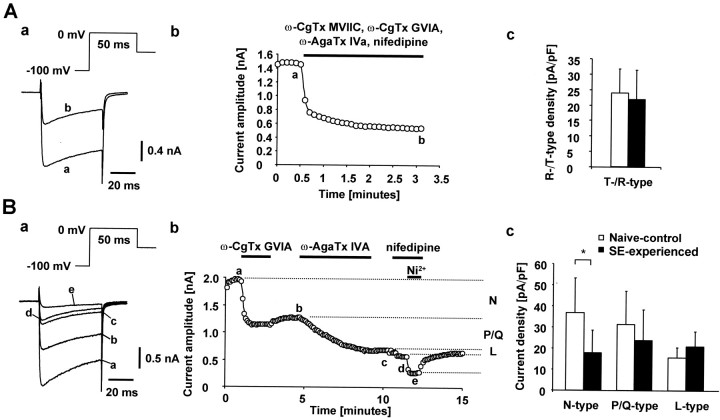
Fig. 8.
Functional analysis of high-threshold current components. A, Recordings were obtained in a dissociated CA1 pyramidal neuron from a naive-control animal. Ca2+ currents were evoked with the voltage paradigm shown in the inset. To isolate the T-/R-type Ca2+ current, the organic blockers ω-conotoxin GVIA (2 μm), ω-conotoxin MVIIC (3 μm), ω-agatoxin GIVA (200 nm), and nifedipine (10 μm) were co-applied, unmasking a rapidly inactivating residual component (a). The current traces in a were recorded at the time points indicated by the lowercase letters inb. Pharmacologically isolated T-/R-type Ca2+ currents were compared in dissociated CA1 pyramidal neurons from SE-experienced (n = 21 cells) and naive-control animals (n = 20 cells) (c). Current densities were obtained by normalizing current amplitudes to cell capacitance. B, In another dissociated neuron from a naive-control animal, the organic Ca2+ channel blockers ω-conotoxin GVIA (2 μm), ω-agatoxin IVa (200 nm), and nifedipine (10 μm) were added sequentially (a; duration of application is indicated byhorizontal bars, b). This allowed assessment of the individual contributions of N-, P/Q-, and L-type Ca2+ current components to the whole-cell current (indicated at right margin of b). Thecurrent traces in a were recorded at thetime points indicated by the lowercase letters in b. High-voltage-activated Ca2+ current densities in naïve control (n = 9–10) versus SE-experienced rats (n = 10) are shown in c. The N-type current density was significantly reduced (*p < 0.05), whereas the density of P/Q- and L-type currents was unaltered.