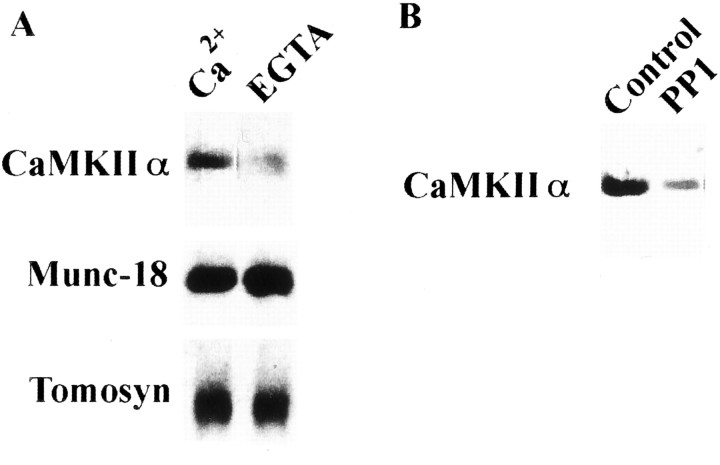
Fig. 3.
Dissociation of the autophosphorylated CaMKII from syntaxin 1A is regulated by decrease of [Ca2+] (A) and its dephosphorylation (B). In both A andB, anti-CaMKIIα (top) and anti-autophosphorylated CaMKIIα (bottom) antibodies were used in immunoblots. Purified rat brain CaMKIIα was autophosphorylated and incubated with immobilized GST–syntaxin as described above (A). Protein complex was additionally incubated with EGTA-treated buffer (see Materials and Methods) for 1 hr at 4°C (A) or with PP1 for 30 min at 25°C (B). Bound CaMKIIα was then eluted with PreScission protease and analyzed using anti-CaMKIIα mAb. EGTA (A) or CaMKII dephosphorylated by PP1 (B) after binding causes CaMKIIα release from syntaxin. Under these conditions, retained CaMKII accounted for only 18.7 ± 1.5% (A) and 14.3 ± 2.3% (B) of the control, respectively (four independent experiments each). In A, Munc-18 and tomosyn were compared with CaMKII under the same experimental conditions, and their binding to syntaxin was completely independent of Ca2+.