Fig. 6.
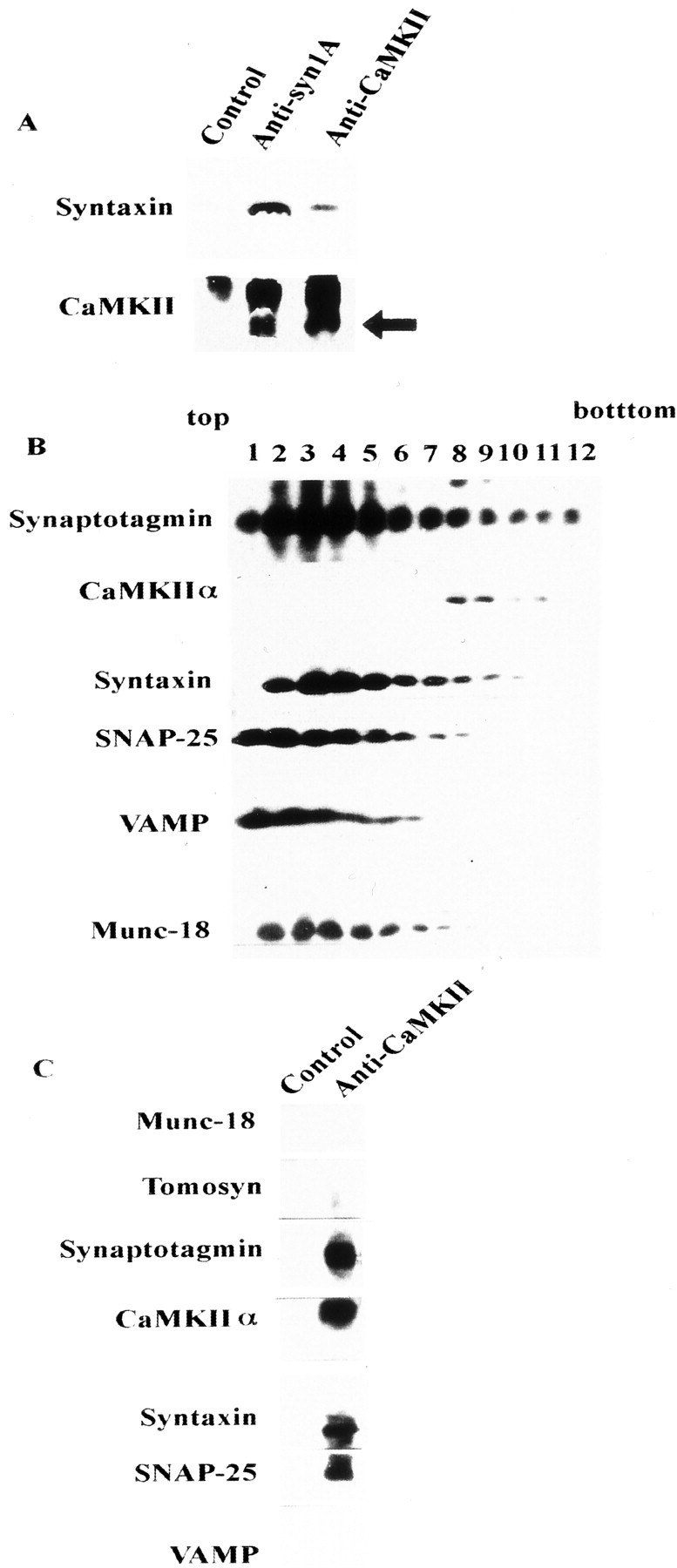
Relationship between the CaMKII–syntaxin complex and other syntaxin-binding proteins. A, Immunoprecipitation of Triton X-100-solubilized synaptosomal proteins using anti-CaMKII or anti-syntaxin antibodies. Complexes were immunostained using anti-syntaxin 1A (top) and anti-CaMKIIα (bottom) mAbs. The arrowindicates the position of CaMKII (the top bandrepresents the IgG heavy chain). Normal mouse IgG was used as the control. Each antibody was incubated with protein G-Sepharose at 4°C for 2 hr, and 10 mg of solubilized synaptosomal proteins (Pevsner et al., 1994; Igarashi et al., 1997) was then added to each antibody–protein G–Sepharose complex at 4°C for an additional 2 hr. Protein–antibody complex eluted with SDS sample buffer was loaded onto 15% SDS-PAGE and immunoblotted against these antibodies. B, Fractionation of synaptosomal proteins solubilized by 1% Triton X-100 and separated on a 10–35% glycerol gradient, as described previously (Igarashi et al., 1997). Fractions (1 ml) were collected from the top. One milliliter of solubilized synaptosomal proteins (protein content was ∼1 mg) was loaded onto 10 ml of a 10–35% continuous glycerol gradient and centrifuged at 41,000 rpm for 17 hr. Fractions (100 μl each) were resolved on 15% SDS-PAGE, and the distribution of each protein was analyzed by immunoblotting. C, Immunoprecipitation of solubilized proteins in fraction 8 of B, which contained both CaMKII and syntaxin, proceeded as described previously (Igarashi et al., 1997, 2000).
