Abstract
Tubulin forms the microtubule and regulates certain G-protein-mediated signaling pathways. Both functions rely on the GTP-binding properties of tubulin. Signal transduction through Gαq-regulated phospholipase Cβ1(PLCβ1) is activated by tubulin through a direct transfer of GTP from tubulin to Gαq. However, at high tubulin concentrations, inhibition of PLCβ1 is observed. This report demonstrates that tubulin inhibits PLCβ1 by binding the PLCβ1 substrate phosphatidylinositol 4,5-bisphosphate (PIP2). Tubulin binding of PIP2 was specific, because PIP2 but not phosphatidylinositol 3,4,5-trisphosphate, phosphatidylinositol 3-phosphate, phosphatidylinositol, phosphatidylcholine, phosphatidylethanolamine, or inositol 1,4,5-trisphosphate inhibited microtubule assembly. PIP2 did not affect GTP binding or GTP hydrolysis by tubulin. Muscarinic agonists promoted microtubule depolymerization and translocation of tubulin to the plasma membrane. PIP2 augmented this process in both Sf9 cells, containing a recombinant PLCβ1 pathway, and SK-N-SH neuroblastoma cells. Colocalization of tubulin and PIP2 at the plasma membrane was demonstrated with confocal laser immunofluorescence microscopy. Although tubulin bound to both Gαq and PLCβ1, PIP2 facilitated the interaction between tubulin and PLCβ1 but not that between tubulin and Gαq. However, PIP2 did augment formation of tubulin–Gαq–PLCβ1 complexes. Subsequent to potentiating PLCβ1 activation, sustained agonist-independent membrane binding of tubulin at PIP2- and PLCβ1-rich sites appeared to inhibit Gαq coupling to PLCβ1. Furthermore, colchicine increased membrane-associated tubulin and also inhibited PLCβ1 activity in SK-N-SH cells. Thus, tubulin, depending on local membrane concentration, may serve as a positive or negative regulator of phosphoinositide hydrolysis. Rapid changes in membrane lipid composition or in the cytoskeleton might modify neuronal signaling through such a mechanism.
Keywords: tubulin, phospholipid, microtubule, cytoskeleton, G-protein, phospholipase C, muscarinic receptor, acetylcholine, G-protein-coupled receptor, calcium, protein kinase C
The phosphatidylinositol 4,5-bisphosphate (PIP2)-specific phospholipase C (PLC) enzymes transduce signals by generating two second messengers, inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. The β isoforms of these enzymes, PLCβ1–3, are activated by the α subunit of the G-protein Gq (for review, see Rhee and Bae, 1997). Among the G-protein-coupled receptors linked to activation of Gαq are the m1, m3, and m5 muscarinic receptor subtypes.
The microtubule protein tubulin is involved in the control of G-protein-mediated signal transduction (Wang et al., 1990; Popova et al., 1994; Roychowdhury and Rasenick, 1994; Ravindra et al., 1996; Cote et al., 1997a,b). Association of tubulin with certain Gα subunits and the subsequent regulation of adenylyl cyclase and PLCβ1 signaling has been reported (Popova et al., 1994, 1997; Yan et al., 1996, 2001; Popova and Rasenick, 2000). After m1 muscarinic receptor stimulation in vitro (Popova et al., 1997) and in vivo (Popova and Rasenick, 2000), cytosolic tubulin translocates to the plasma membrane. Membrane-associated tubulin regulates PLCβ1activation both in a positive and negative manner (Popova et al., 1997). At low (nanomolar) concentrations, tubulin activates PLCβ1, whereas at higher concentrations, enzyme inhibition is observed.
Previous studies have indicated that transactivation of Gαq, through a direct GTP transfer from tubulin, is responsible for PLCβ1 activation by tubulin (Popova et al., 1997; Popova and Rasenick, 2000). However, the mechanism behind the inhibition of PLCβ1, observed at high dimeric tubulin concentrations, has not been elucidated.
Tubulin binds PIP2, and this inhibits microtubule polymerization (Popova et al., 1997). Because PIP2 is the PLC preferred substrate, sequestration of PIP2 by tubulin should also affect important phosphoinositide-dependent signaling pathways. By analogy, several actin-binding proteins, such as profilin, gelsolin, and CapG, have already been shown to bind PIP2and to modulate the activity of regulatory PLC isozymes both in vitro (Goldschmidt-Clermont et al., 1990, 1991; Banno et al., 1992; Steed et al., 1996; Sun et al., 1997) and in vivo (Sun et al., 1997). The binding of PIP2 by the above-mentioned proteins appears to prevent phospholipase access to this substrate (Goldschmidt-Clermont et al., 1990, 1991; Banno et al., 1992; Steed et al., 1996; Sun et al., 1997). Because high tubulin concentrations inhibit PLCβ1 in vitro, a similar inhibitory mechanism was suggested (Popova et al., 1997).
This study was designed to evaluate the interaction between tubulin and PIP2 and test how this interaction affects Gαq and PLCβ1 activation at the membrane. The results reveal that PIP2 binding to tubulin is specific but does not affect the binding and hydrolysis of GTP by tubulin. Although activated muscarinic receptors recruit tubulin from the cytosol to the membrane, leading to Gαq transactivation, receptor-independent binding of tubulin to PIP2-rich sites on the membrane obstructs PLCβ1 activation. Thus, it appears that tubulin and PIP2 interact to effect a dual regulation of PLCβ1. Such a mechanism might prove important in regulating the response and responsiveness of G-protein-mediated phospholipid signaling in neuronal and glial cells.
MATERIALS AND METHODS
Baculovirus-directed expression of signaling proteins in Sf9 cells. Sf9 cells were maintained in Sf-900 II SFM media (Invitrogen, Carlsbad, CA) as described previously (Popova et al., 1997). They were infected with baculoviruses bearing the m1 muscarinic receptor, Gαq, or PLCβ1cDNAs, as was done previously (Popova et al., 1997). The construction of recombinant baculoviruses was already reported previously (Parker et al., 1991; Graber et al., 1992; Boguslavsky et al., 1994). Cells were harvested after 65 hr, and membranes were prepared and frozen in liquid nitrogen for subsequent use as described previously (Popova et al., 1994, 1997). Protein concentrations were determined by Coomassie blue binding (Bradford, 1976). Bovine serum albumin was used as a standard. Protein expression was measured by immunoblotting. Antisera specific for the m1 muscarinic receptor (number 71; from G. Luthin, MCP Hahnemann University, Philadelphia, PA), Gαq/11 (number 0945; from D. Manning, University of Pennsylvania, Philadelphia, PA), and PLCβ1 (anti-holoenzyme; from S. G. Rhee, National Institutes of Health, Bethesda, MD) were used at a dilution of 1:500. Biotinylated goat anti-rabbit IgG or anti-mouse IgG and streptavidin-alkaline phosphatase conjugate were used for detection. Densitometry was performed to evaluate the expression levels (Storm 840; Molecular Dynamics, Sunnyvale, CA; Popova et al., 1997; Popova and Rasenick, 2000). m1 muscarinic receptor density was determined by [3H]l-quinuclidinyl [phenyl-4(n)]benzilate ([3H]QNB) binding (Popova et al., 1997).
Tubulin preparations. Microtubule proteins were isolated (Shelanski et al., 1973), and tubulin preparations purified free of microtubule-associated proteins by phosphocellulose chromatography were prepared as described previously (Wang and Rasenick, 1991). Phosphocellulose-purified tubulin (PC-tubulin) was >95% pure as determined on SDS-PAGE.
P3(4-azidoanilido)-P1–5′-GTP (AAGTP) and [32P]AAGTP were synthesized as described previously (Rasenick et al., 1994). Tubulin–[32P]AAGTP was made from PC-tubulin as indicated (Rasenick and Wang, 1988). The final preparations contained 0.4–0.6 mol of nucleotide bound/mol of tubulin. Tubulin–[32P]AAGTP concentrations used throughout the study were based on the protein concentration.
To prepare tubulin labeled covalently with fluorescein-5-maleimide (FM-tubulin), FM (Molecular Probes, Eugene, OR) was incubated with PC-tubulin at a 5:1 molar ratio at 37°C for 30 min in polymerization buffer [100 mm 1,4-piperazinediethanesulfonic acid (Pipes), 2 mm EGTA, 4 mmMgCl2, 1 mm GTP, pH 6.9, and 1m glutamate]. The reaction was quenched with 1 mm β-mercaptoethanol, and the samples were layered onto warm 40% sucrose containing 1 mm GTP and centrifuged at 200,000 × g at 37°C for 30 min. The FM-tubulin pellet was washed twice with warm buffer and depolymerized on ice, followed by chromatography through a P6-DG column (Bio-Rad, Hercules, CA) twice to remove free FM. The calculated ratio of FM labeling of tubulin was 1:1. FM-tubulin was polymerization-competent as tested by electron microscopy performed as described previously (Popova et al., 1997).
Microtubule assembly. To test the effects of various phospholipids on microtubule assembly, phosphocellulose-purified tubulin (1.5 mg/ml) was incubated in a bath sonicator for 15 min at 4°C with different phosphoinositides, IP3, or heparin (as indicated), at a molar ratio of 1:6 in polymerization buffer (in mm: 100 Pipes, 2 EGTA, 3 MgCl2, and 1 GTP, pH 6.9). The assembly reaction was performed for 1 hr at 37°C in a shaking water bath. The polymer mass was isolated by centrifugation at 150,000 × g for 30 min at 37°C, followed by separation of the pellets and the supernatants. Pellets were resuspended in identical amounts of cold polymerization buffer on ice, and protein concentrations were measured by the method of Bradford (1976) using BSA as a standard. The amount of protein in the pelleted polymer mass without any additions (control) was 0.47 ± 0.10 mg/ml. The depolymerized pellets were subjected to SDS-PAGE and immunoblotting with a monoclonal anti-α-tubulin antibody (Santa Cruz Biotechnology, Santa Cruz, CA) and ECL detection (Amersham Biosciences, Piscataway, NJ). The results were analyzed in a Storm 840 imaging system (Molecular Dynamics). Samples from microtubule polymerization reactions were also examined by electron microscopy (Popova et al., 1997) to evaluate the effect of lipids on microtubule assembly.
To study whether the effect of PIP2 on microtubule assembly was concentration-dependent, polymerization of phosphocellulose-purified tubulin (2.5 mg/ml) was monitored by turbidity measurement at 350 nm in a Beckman DU 640B spectrophotometer at 37°C. PIP2, phosphatidylcholine (PC), or vesicles of PIP2 and PC (at a molar ratio of 1:1) were mixed with tubulin (tubulin/lipid molar ratio of 1:6) in polymerization buffer on ice to a final volume of 300 μl. Samples were transferred to a quartz cuvette, and the increase in absorbance was monitored at 37°C.
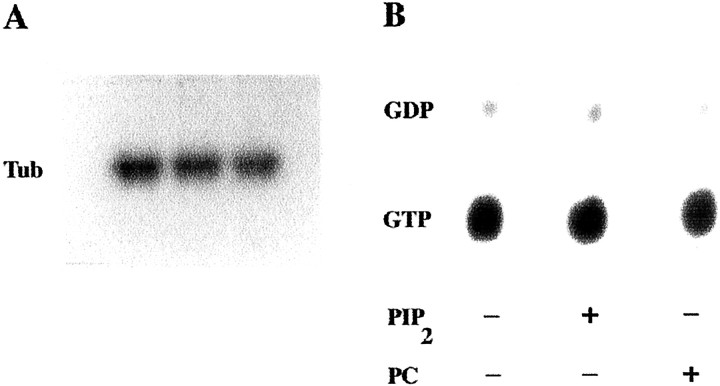
GTPase activity of tubulin. To determine the amount or the species of the guanine nucleotide bound to tubulin and the extent of GTP hydrolysis, phosphocellulose-purified tubulin was made nucleotide-free by incubation with charcoal (Rasenick and Wang, 1988). This tubulin was then incubated with 0.2 mm[32P]GTP for 30 min on ice. After two passes through Bio-Gel P6-DG columns to remove unbound nucleotide, tubulin GTPase activity was determined. Tubulin–[32P]GTP was incubated for 30 min at 30°C, followed by nucleotide analysis by thin-layer chromatography (TLC) on polyethyleneimide cellulose as described previously (Roychowdhury and Rasenick, 1994). The chromatograms were developed in 0.35 mNH4HCO3. The spots containing GTP or GDP standards were visualized with a UV lamp, and the plate was exposed to film for autoradiography or subjected directly to phosphorimage analysis (Storm 840; Molecular Dynamics). When indicated, phosphoinositides (at a molar ratio of 6:1) were added to tubulin–[32P]GTP before incubation (Popova et al., 1997).
Analysis of nucleotide bound to tubulin.Phosphocellulose-purified tubulin was loaded with [32P]AAGTP or [32P]GTP, as described above, in the presence or absence of phosphoinositides (tubulin/phosphoinositide ratio of 1:6). [32P]AAGTP-labeled samples were subjected to TLC, followed by autoradiography or phosphorimage analysis, as indicated. Tubulin samples labeled by [32P]GTP were subjected to P6-DG column chromatography, and the radioactivity of 5 μl of each tubulin–[32P]GTP eluate was measured by liquid scintillation counting.
To test whether PIP2 caused dissociation of the guanine nucleotide bound to tubulin, PIP2 was added at the end of the binding reaction. The samples were kept on ice for an additional 30 min before being processed as described above.
Phosphoinositide preparation. Phosphoinositides were evaporated under a stream of nitrogen, sonicated for 5 min (at appropriate concentrations) in assay buffer on ice, and used immediately.
Photoaffinity labeling. Membranes from Sf9 or SK-N-SH cells were incubated with the indicated concentrations of tubulin–[32P]AAGTP, PIP2, and carbachol as described previously (Popova et al., 1994, 1997). After UV irradiation and centrifugation, membrane pellets were dissolved in Laemmli buffer and subjected to SDS-PAGE as done previously (Popova et al., 1994, 1997). Gels were either stained (Coomassie blue) or subjected to Western blotting, followed by autoradiography (XAR-5 film; Eastman Kodak Co., Rochester, NY) or phosphorimaging. Densitometric measurements of autoradiograms and phosphorimage analysis of the gels were performed, respectively (Storm 840; Molecular Dynamics). Tubulin–[32P]AAGTP and Sf9 membranes, overexpressing Gαq, were run along the samples to identify the bands of tubulin and Gαq. As shown previously (Popova and Rasenick, 2000), carbachol-evoked membrane association of tubulin and Gαq transactivation by tubulin were consistently reversed by atropine.
Immunoprecipitation. Sf9 cells were infected separately or simultaneously (according to the experimental design) with baculoviruses bearing the m1 muscarinic receptor, Gαq, or PLCβ1 cDNA as described previously (Popova et al., 1997). Membrane preparations were extracted with 1% sodium cholate for 1 hr at 4°C and constant stirring (Popova et al., 1997). After centrifugation at 20,000 ± g at 4°C the extracts (0.5 mg/ml membrane protein) were incubated with 1 μm of tubulin–[32P]AAGTP as described previously (Popova et al., 1997). When tested, PIP2 was preincubated with tubulin–[32P]AAGTP at a molar ratio of 6:1 for 15 min in a Branson (Danbury, CT) water bath sonicator at 4°C. After UV irradiation and preclearing with Pansorbin (Calbiochem, La Jolla, CA), each sample was incubated overnight with appropriate antiserum or preimmune serum (1:20 dilution) at 4°C with constant shaking. Immune complexes were precipitated with Pansorbin and subjected to SDS-PAGE and autoradiography or phosphorimage analysis. The antisera used showed no cross-reactivity to tubulin.
Analysis of phosphoinositide hydrolysis in SK-N-SH cells.SK-N-SH neuroblastoma cells were grown in six-well plates in DMEM supplemented with 10% fetal bovine serum and 50 U/ml penicillin-streptomycin. Twenty-four hours before the experiment, inositol-free DMEM supplemented with 2 μCi/wellmyo-[3H]inositol was added. The cells were washed three times with Locke's buffer, containing 10 mm LiCl, and incubated for 15 min with or without 33 μm colchicine in the same buffer. After triplicate wash with Locke's buffer, 10 μmcarbachol was added as indicated, and the cells were incubated for 30 min at 37°C. Carbachol effects were routinely controlled for by addition of 1 μm atropine. The reaction was stopped with ice-cold 10% trichloroacetic acid, and the cells were scraped from wells with a rubber policeman and transferred to tubes. After sonication (as described above) and centrifugation at 20,000 × g for 15 min (4°C), the supernatants were extracted with water-saturated ether and neutralized with 1mNH4HCO3. Ion exchange chromatography (Dowex AG 1-X8 resin, formate form; Bio-Rad) of the samples was performed as described previously (Popova and Dubocovich, 1995). Total [3H]inositol phosphates were quantified by liquid scintillation counting. The inositol phosphate content of SK-N-SH cells at the start of the experiment (0% increase) was 1.1 ± 0.27 × 103dpm/106 cells.
Recording of enhanced green fluorescent protein-tubulin-containing SK-N-SH cells by immunofluorescence microscopy. Cells plated on 35-mm-diameter Delta T dishes (Biotechs, Inc.) were transiently transfected with 5 μg enhanced green fluorescent protein (EGFP)-tubulin cDNA (Clontech, Cambridge, UK) using Lipofectin reagent as described by the manufacturer (Invitrogen, Gaithersburg, MD). The cells were observed 24 hr later using fluorescence microscopy. A Nikon fluorescence microscope equipped with a 100 W mercury arc lamp was used. Before observation, the medium in the dish was changed to serum-free DMEM containing 20 mmHEPES, and the cells were maintained in this media for at least 30 min before the recording. The cells were transferred to the microscope stage and maintained at 37°C during the entire period of observation. Images were acquired with an interline charge-coupled device camera (1300 YHS; Roper Scientific, Trenton, NJ) driven by IP Lab imaging software (Scananlatics, Inc., Suitland, VA). Fluorescent images for EGFP were recorded every 15 sec and the recorded images were processed with IP Lab.
Confocal immunofluorescence microscopy. SK-N-SH neuroblastoma cells were plated onto glass coverslips in 12-well culture plates at a density of 1 × 105. After 24 hr, cells were incubated for 15 min with or without 33 μm colchicine. After a PBS wash, the cells were treated for 2 min with 1 mm carbachol, 10 μm atropine, or both. The cells were immediately fixed in −20°C methanol for 3 min and washed three times, 10 min each, in PBS containing 0.1% Triton X-100. The cells were blocked for 40 min in PBS containing 5% milk and washed in PBS. Subsequently the cells were incubated for 1 hr with a polyclonal anti-tubulin antibody (raised against the C-terminal 422–431 amino acid region of β-tubulin;Popova and Rasenick, 2000) and a monoclonal anti-PIP2 antibody (Assay Designs, Inc.), both at a dilution of 1:100. After a PBS wash, secondary fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit and Texas Red-conjugated horse anti-mouse antibodies (Vector Laboratories, Burlingame, CA; 1:100 dilution) were applied for 1 hr, followed by washing and mounting of the coverslips. Images were acquired using aZeiss (Thornwood, NY) LSM 510 laser scanning confocal microscope equipped with a 63× water immersion objective. A 488 nm beam from an argon-krypton laser was used for the excitation of FITC, whereas a 543 nm beam was used for Texas Red excitation. Emission from FITC was detected through a BP505 filter, whereas emission from Texas Red was detected through an LP560 filter. Areas of antibody colocalization appeared in yellow. Differential interference contrast images of the cells were regularly acquired as well. Coverslips were examined at random. For each experimental condition, a total of 90 randomly selected cells over three consecutive experiments were evaluated for tubulin and PIP2 distribution and colocalization. Final image composites were created using Adobe Photoshop 5.0. No specific FITC or Texas Red labeling was observed in cells treated with rabbit or mouse preimmune serum instead of anti-β-tubulin or anti-PIP2 antibodies, respectively. FITC labeling was not observed when the anti-tubulin antiserum was preincubated overnight at 4°C with PC-tubulin (1:1 ratio), and Texas Red labeling was not detected when the anti-PIP2 antiserum was preincubated with PIP2 (1:1 ratio), both conditions tested at the same antibody dilutions (1:100) afterward. Although colchicine treatment changed the shape of the treated cells, it did not affect the membrane localization and intracellular distribution of Gαq (Ibarrondo et al., 1995).
Materials. [α32P]GTP was from ICN Biomedicals (Cleveland, OH). [3H]QNB was from Amersham Biosciences. Carbachol and all phosphoinositides used were from Sigma (St. Louis, MO). Fluorescein-5-maleimide was from Molecular Probes.p-Azidoaniline was synthesized by Dr. William Dunn III (University of Illinois at Chicago). All other reagents were of analytical grade.
RESULTS
Specific interaction with PIP2 decreases tubulin polymerization
Previous experiments demonstrated that PIP2bound to tubulin and inhibited microtubule assembly (Popova et al., 1997). The specificity of tubulin–PIP2interaction was not addressed in that study. Phosphatidylinositol 3,4,5-trisphosphate (PIP3) as well as the second messenger IP3 can bind to certain PIP2-binding protein domains (Ferguson et al., 1995; Kavran et al., 1998), and the negative charge of these molecules was presumed responsible for such interaction. The anionic phospholipid constituents of hepatic membranes have also been reported to account for membrane binding of brain microtubule protein and inhibition of assembly (Reaven and Azhar, 1981). A hydrophobic interaction of tubulin (Andreu, 1986) with the uncharged phospholipid phosphatidylcholine at the lipid phase transition temperature (Klausner et al., 1981; Kumar et al., 1981) has been found as well.
To investigate the specificity of tubulin interaction with the anionic phospholipid PIP2, several charged and neutral phospholipids as well as IP3 were included in microtubule polymerization assays. PC-tubulin, purified free of microtubule-associated proteins, was preincubated with the phospholipids tested or IP3. These tubulin preparations were allowed to polymerize under conditions that favor microtubule assembly (see Materials and Methods). In each case, the amount of tubulin distributed between the pelleted polymer mass and the supernatant was measured. The results obtained demonstrated that PIP2 inhibited tubulin polymerization by 39.9 ± 3.6% (SEM; n = 5) compared with the control not containing this phosphoinositide (Fig.1A). Other closely related anionic phosphoinositides, such as PIP3, phosphatidylinositol 3-phosphate (PIP), and phosphatidylinositol (PI), as well as the negatively charged inositol phosphate IP3, had no significant effect on the microtubule assembly process. When tested under the same conditions, the polyanion heparin also had no effect on tubulin assembly. The neutral phospholipids PC and phosphatidylethanolamine (PE) did not significantly affect polymerization either (Fig. 1A). Electron microscopy and light scattering of tubulin samples was also done. As was the case with microtubule pellets, PIP2 but not PIP3, PC, PE, PI, or IP3 inhibited microtubule formation. Thus, it is suggested that the regulatory phosphoinositide PIP2 inhibits microtubule polymerization through a specific interaction with tubulin.
Fig. 1.
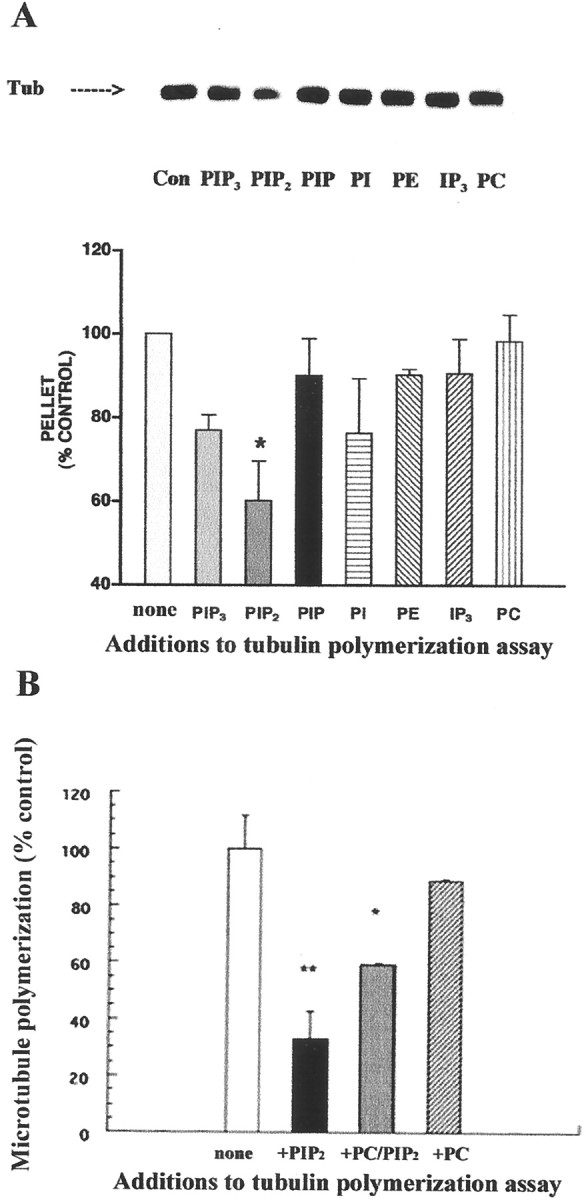
PIP2 inhibits tubulin polymerization.A, Comparison of the effects of various phosphoinositides on microtubule polymerization. Where indicated, PIP3, PIP2, PIP, PI, PC, PE, and the inositol phosphate IP3 (final molar concentration of 75 μm) were preincubated with tubulin (Tub) as described in Materials and Methods. Microtubule polymerization reactions were performed for 1 hr at 37°C. Pellets were resuspended in cold polymerization buffer and subjected to SDS-PAGE and immunoblotting with a monoclonal anti-α tubulin antibody. Values are means ± SEM of five independent experiments performed in triplicate. *Significantly different from the control (tubulin, subjected to polymerization without any addition);p < 0.05, one-way ANOVA. Colorimetric measurements of the protein content of depolymerized pellets (performed before SDS-PAGE) corroborated these findings. B, PIP2 inhibition of microtubule formation is concentration-dependent. PIP2, PC, mixed vesicles of PIP2 and PC (at the ratio of 1:1), and vehicle were added to microtubule polymerization reactions. Polymerization was performed for 30 min as described in Materials and Methods. Absorbance at 350 nm was monitored. Values were obtained after 20 min, when the polymerization reactions were at equilibrium. Values are means ± SEM of three separate experiments done in triplicate. The net absorbance of microtubule polymerization reactions without added phosphoinositides was 1.09 ± 0.12 (control). *p < 0.05; **p < 0.01.
To test whether the effect of PIP2 on tubulin was concentration-dependent, tubulin polymerization was studied in the absence or presence of PIP2, PC, or the mixture of both in light-scattering experiments (Fig. 1B). When the concentration of PIP2 was reduced by half (PIP2 mixed with PC at a molar ratio of 1:1), the inhibition of microtubule formation was also half that seen with PIP2 alone.
PIP2 does not affect the binding and hydrolysis of GTP by tubulin
Several possible mechanisms exist through which PIP2 binding could interfere with tubulin polymerization. One possibility is that PIP2affects the binding of GTP to tubulin, because PIP2 has been reported to promote dissociation of GTP from the small GTP-binding proteins Arf, CDC 42, and Rho (Terui et al., 1994; Glaven et al., 1996). Another scenario is that GTP hydrolysis on tubulin is activated by PIP2. Because tubulin must bind GTP to assemble, PIP2could block the process by activating tubulin GTPase.
PIP2 did not modify the amount of [32P]AAGTP or [32P]GTP bound to tubulin, estimated at 0.49 ± 0.08 mol bound/mol of tubulin (Fig.2A). This was independent of whether PIP2 was added to tubulin before or after the course of the guanine nucleotide binding reaction. Densitometry revealed relative absorbance values of 100.0 ± 14.6 for the tubulin–[32P]AAGTP band obtained when PIP2 was not present in the binding reaction, as well as 94.41 ± 10.2 and 103.3 ± 15.1 for the bands obtained when PIP2 was added before or after the binding reaction, respectively (p > 0.05; n = 6 for each experimental condition).
Fig. 2.
PIP2 does not alter binding and hydrolysis of GTP by tubulin. A, Effects of PIP2 on [32P]AAGTP binding to tubulin (Tub). Phosphocellulose-purified tubulin, stripped of nucleotide, was incubated with [32P]AAGTP in the absence or presence of phosphoinositides. First lane, No addition to the binding reaction; second lane, PIP2 added at the start of the binding reaction; third lane, PIP2 added after the end of the binding reaction, as described. A representative of two identical experiments performed in triplicate with similar results is shown. B, Effects of PIP2 on GTPase activity of tubulin. Tubulin–[32P]GTP was incubated for 30 min at 30°C, in the absence or presence of phosphoinositides, and the nucleotide bound to tubulin was analyzed as described in Materials and Methods. A representative of two identical experiments performed in triplicate with similar results is shown.
Tubulin contains an intrinsic GTPase, which is not activated until the microtubule is formed (Carlier and Pantaloni, 1981). If PIP2 activated the GTPase of tubulin dimers, polymerization would be blocked, because those dimers would be binding GDP. However, PIP2 did not promote hydrolysis of GTP by tubulin. The amount of GTP bound per mole of tubulin remained at 0.55 ± 0.05 mol/mol during the course of these experiments regardless of the presence or absence of phospholipids (p > 0.05; n = 6 for each experimental condition; Fig. 2B).
PIP2 promotes association of tubulin with the membrane but does not promote Gαq transactivation by tubulin
PIP2 is normally membrane-associated. Although it is not clear how tubulin associates with membranes (a subject of some controversy), it is possible that PIP2 is involved in the process (Reaven and Azhar, 1981). This was investigated using both membranes prepared from Sf9 cells expressing recombinant m1 muscarinic receptors, Gαq, and PLCβ1 (Popova et al., 1997) and membranes from SK-N-SH neuroblastoma cells, which normally contain m3 muscarinic receptors, Gαq, and PLCβ1 (Fisher and Heacock, 1988).
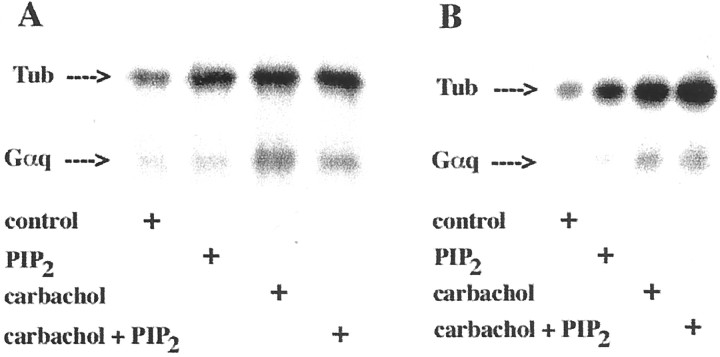
In membranes from infected Sf9 or SK-N-SH cells, both carbachol and PIP2 increased the association of tubulin with the membrane (Fig. 3). At the experimental conditions used (Fig. 3A), the average increase in association of tubulin–[32P]AAGTP with the Sf9 cell membranes was 99.6 ± 22.4% (SD;n = 3) in the presence of carbachol and 96.1 ± 17.7% (n = 3) in the presence of PIP2. When both of them were present, association of tubulin with the membrane was 115.1% ± 29.2% (n = 3) greater than that of the control. Comparable results were obtained when membranes from SK-N-SH cells were tested (Fig, 3B).
Fig. 3.
PIP2 binding increases membrane-associated tubulin (Tub). A, Membranes from Sf9 cells expressing m1 muscarinic receptors, Gαq, and PLCβ1 (20 μg of membrane protein) were incubated with 300 nm tubulin–[32P]AAGTP with or without 100 μm carbachol, 30 μmPIP2, or both for 5 min at 23°C, followed by UV irradiation, SDS-PAGE (50 μg of membrane protein in eachlane), and autoradiography. A representative of three similar experiments performed in triplicate is shown. B, Membranes from SK-N-SH cells (40 μg of membrane protein) were incubated with tubulin–[32P]AAGTP or carbachol, PIP2, or both under the conditions described above. A representative of two identical experiments performed in triplicate with similar results is shown.
Gαq activation by tubulin was also assessed in these experiments by examining the transfer of [32P]AAGTP from tubulin to Gαq. Although Gαq transactivation by tubulin increased by 124.0 ± 23.0% (SD; n = 3) after muscarinic receptor stimulation (Fig. 3A), it was not affected by PIP2. Atropine inhibited the membrane association of tubulin evoked by carbachol, but it failed to suppress the PIP2-promoted membrane association of tubulin. These findings were corroborated when SK-N-SH membranes were tested under similar experimental conditions (Fig.3B).
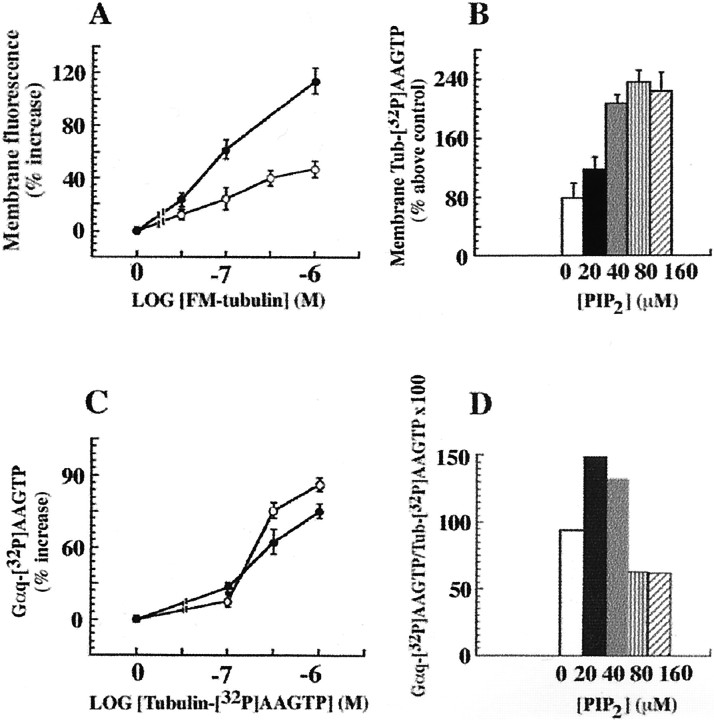
Concentration–response experiments were performed to inspect the effect of PIP2 on tubulin regulation of Gαq. PIP2 increased the binding of exogenous tubulin to Sf9 membranes containing the recombinant proteins over a range of tubulin concentrations. Both FM-tubulin (Fig.4A) and tubulin–[32P]AAGTP gave similar results. The effects of PIP2 on tubulin–[32P]AAGTP membrane association were also concentration-dependent (Fig. 4B). However, as shown in Figure 4C, over a range of tubulin–[32P]AAGTP concentrations, transactivation of Gαq by tubulin was independent of PIP2. In fact, a decrease in the carbachol-evoked [32P]AAGTP transfer from tubulin to Gαq was observed at PIP2 concentrations of >40 μm (Fig. 4D). Thus, although PIP2 promoted tubulin association with the membrane, it did not evoke the rapid process of Gαq transactivation by tubulin. These results are consistent with the notion that PIP2 binding to tubulin interfered with both tubulin polymerization properties and the ability to transactivate Gαq.
Fig. 4.
PIP2 does not increase Gαq transactivation by tubulin. A, The effects of carbachol and PIP2 on the membrane association of tubulin are additive. Recruitment of tubulin to the membrane was studied using increasing concentrations of FM-tubulin. Membranes from Sf9 cells expressing m1 muscarinic receptors, Gαq, and PLCβ1 were incubated with carbachol and FM-tubulin (at the indicated concentrations), with or without PIP2. SDS-PAGE (50 μg of membrane protein in each lane) was followed by measurement of the fluorescence of membrane-associated tubulin with a fluorescence imaging system (Storm 840; Molecular Dynamics). The results represent one of three similar experiments performed in triplicate. Open circles, Membranes treated with 1 mm carbachol; filled circles, membranes treated with 1 mm carbachol and 30 μmPIP2. B, PIP2-assisted recruitment of tubulin–[32P]AAGTP to the membrane is concentration-dependent. Membranes from Sf9 cells containing m1 muscarinic receptors, Gαq, and PLCβ1 were incubated with 1 mm carbachol, 1 μmtubulin–[32P]AAGTP, and increasing concentrations of PIP2, as described in Figure 1. After SDS-PAGE (50 μg of membrane protein in each lane)32P-labeled protein bands were measured by phosphorimage analysis. One of three identical experiments done in triplicate with similar results is shown. C, Carbachol-evoked Gαq transactivation by tubulin is not affected by PIP2. The experiments were done as described in A, except that tubulin–[32P]AAGTP was used. Proteins were resolved by SDS-PAGE (50 μg of membrane protein in each lane), and the radioactivity of the Gαq bands ([32P]AAGTP was transferred from tubulin) was measured by phosphorimage analysis (Storm 840; Molecular Dynamics). One of five independent experiments done in triplicate with similar results is shown. Open circles, Membranes treated with 1 mm carbachol;filled circles, membranes treated with 1 mmcarbachol and 30 μm PIP2. D, The increased membrane association caused by PIP2 was not linked to Gαq transactivation. The percent ratios of [32P]AAGTP-labeled Gαq and tubulin–[32P]AAGTP at the various PIP2 concentrations are derived from the experiment described in B. One of three identical experiments done in triplicate with similar results is shown. Control values forB and D represent the amount of tubulin associated with the plasma membrane in the absence of carbachol or PIP2.
PIP2 increases the association of tubulin with PLCβ1
Because PIP2 is the natural substrate for PLCβ1, the relevance of PLCβ1 to the process of PIP2-mediated association of tubulin with the membrane was tested. In the absence of PLCβ1, PIP2 had no effect on the association of tubulin with the Sf9 cell membranes (Fig.5A). Thus, it appeared that PLCβ1 was involved in the PIP2-promoted membrane association of tubulin.
Fig. 5.
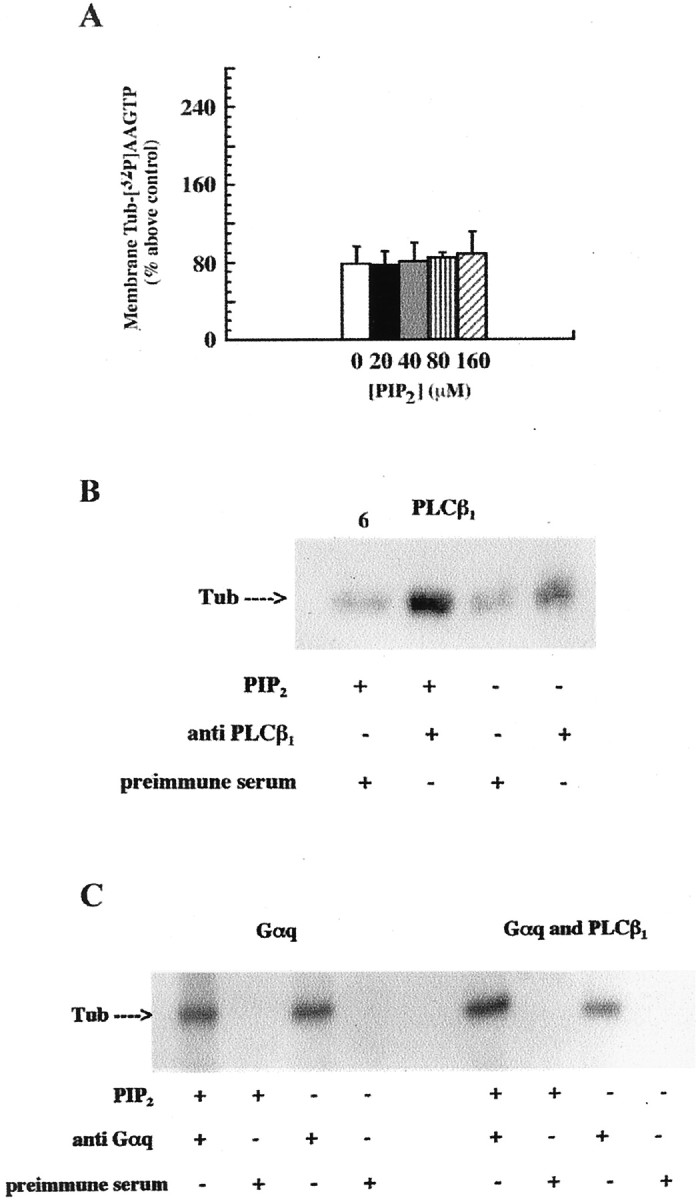
PIP2 is involved in the association of tubulin with PLCβ1. A, PIP2does not potentiate the membrane association of tubulin when PLCβ1 is not present. Membranes from Sf9 cells containing m1 muscarinic receptors and Gαq but not PLCβ1 were incubated with tubulin–[32P]AAGTP and the indicated concentrations of PIP2 as described in Figure4B. One of two identical experiments done in triplicate with similar results is shown. Tubulin associated with the membrane in the absence of carbachol represents the control.B, PIP2 increases coimmunoprecipitation of PLCβ1 with tubulin (Tub). Membrane preparations of Sf9 cells containing PLCβ1 were extracted with 1% sodium cholate. Where indicated, tubulin–[32P]AAGTP was preincubated with PIP2 as described in Materials and Methods. After UV irradiation, each sample was incubated overnight with anti-PLCβ1 antiserum or preimmune serum, as indicated, and immunoprecipitated as described. The immunoprecipitates were subjected to SDS-PAGE and autoradiography. An autoradiogram from one of four independent experiments with identical results is shown.C, PIP2 increased coimmunoprecipitation of Gαq and tubulin when PLCβ1 was present. Membrane preparations of Sf9 cells, expressing either Gαq or Gαq and PLCβ1, were tested as described inB, except that anti-Gαq antiserum was used to test Gαq coimmunoprecipitation with tubulin. Note that Gαq expression level decreased when Sf9 cells were cotransfected with Gαq and PLCβ1 baculoviruses, as revealed by immunoblotting with anti-Gαq antiserum. An autoradiogram from one of three similar experiments is shown.
This was tested by coimmunoprecipitation. Tubulin coimmunoprecipitates with Gαq and, to a lesser extent, PLCβ1(Popova et al., 1997). However, the mechanism whereby PIP2 affects these interactions has not been evaluated. Extracts from Sf9 membranes, containing PLCβ1, Gαq, or both, were tested (Fig.5B,C). PIP2 increased coimmunoprecipitation of tubulin–[32P]AAGTP with PLCβ1 by approximately twofold [204 ± 11.0% (SD)], suggesting stabilization of tubulin–PLCβ1 interaction (Fig.5B). PIP2 did not alter the coimmunoprecipitation of tubulin and Gαq (Fig. 5C,left). However, when Gαq and PLCβ1were both present on the membrane, PIP2 increased Gαq–tubulin coimmunoprecipitation by twofold [216 ± 10.0% (SD); Fig. 5C, right]. These results suggested that PIP2 might promote the formation of tubulin–Gαq–PLCβ1 complexes.
Carbachol stimulation causes redistribution and colocalization of intracellular tubulin with PIP2 at the plasma membrane
If tubulin–PIP2 interaction modulates a related membrane signaling event, we would expect to see colocalization of tubulin and PIP2 at regions of the cell specialized for signaling. To examine this, SK-N-SH cells were transiently transfected with pEGFP–tubulin. Immunofluorescence microscopy was used to confirm in vivo the microtubule depolymerization and redistribution of tubulin in SK-N-SH cells in response to carbachol stimulation. Although the appearance of the microtubules in cells treated with vehicle did not change, rapid microtubule depolymerization was observed in the carbachol-treated cells (Fig. 6).
Fig. 6.
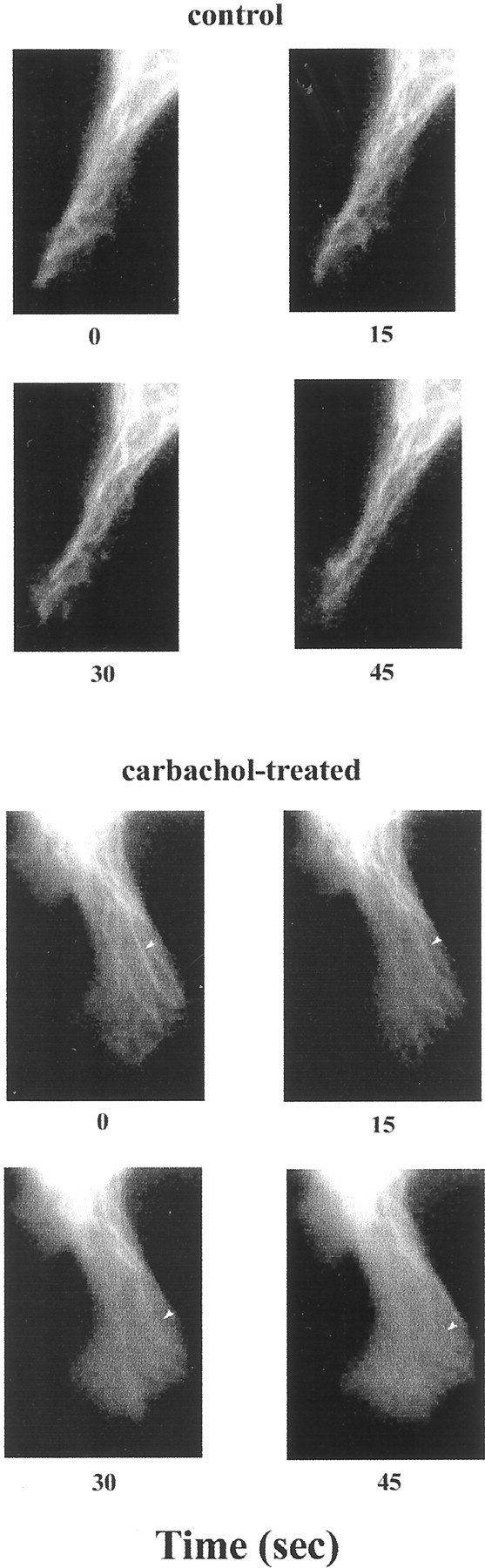
Microtubule depolymerization and redistribution of tubulin in response to carbachol stimulation in GFP–tubulin-expressing SK-N-SH cells. SK-N-SH cells were transfected with EGFP–tubulin cDNA as described. Twenty four hours after transfection, cells treated with either vehicle (control) or 100 μm carbachol were observed on a heated (37°C) microscope stage, and images were collected at 15 sec intervals as described. Arrowheads indicate microtubule depolymerizing in response to carbachol treatment.
Confocal laser immunofluorescence microscopy was used to compare the patterns of localization of tubulin and PIP2 in carbachol-treated and untreated SK-N-SH cells. A monoclonal antibody shown to bind specifically to endogenous PIP2 and to inhibit the intracellular breakdown of this phosphoinositide was used (Fukami et al., 1988). This antibody blocked the PIP2-mediated increase in tubulin binding to isolated SK-N-SH membranes. Because Lipofectin treatment compromised membrane PIP2, EGFP–tubulin-transfected cells could not be used in this study. Anti-tubulin antibody raised against the C-terminal 422–431 amino acid region of β-tubulin was used to visualize tubulin (Popova and Rasenick, 2000). In both carbachol-treated and untreated SK-N-SH cells, anti-PIP2 antibody labeling (seen in red) was detected along the cell surface and in the cytoplasm, but it was mostly enriched in the membrane and submembrane regions of the cell (Fig. 7). In the untreated cells, tubulin (seen in green) was found in microtubules, bundles, and throughout the cytoplasm. Some tubulin colocalized with PIP2 in areas close to the plasma membrane (Fig. 7A, yellow). (Note that because confocal images of cell areas that are 1 μm thick are presented, filamentous microtubule arrays are not obvious.) When SK-N-SH cells were stimulated with carbachol, microtubule depolymerization and redistribution of tubulin along the plasma membrane was observed (Fig. 7B). Tubulin colocalized with PIP2 in regions along the plasma membrane. Tubulin and PIP2 did not colocalize in areas distal to the plasma membrane in control and carbachol-treated cells. All effects of carbachol were blocked by atropine.
Fig. 7.
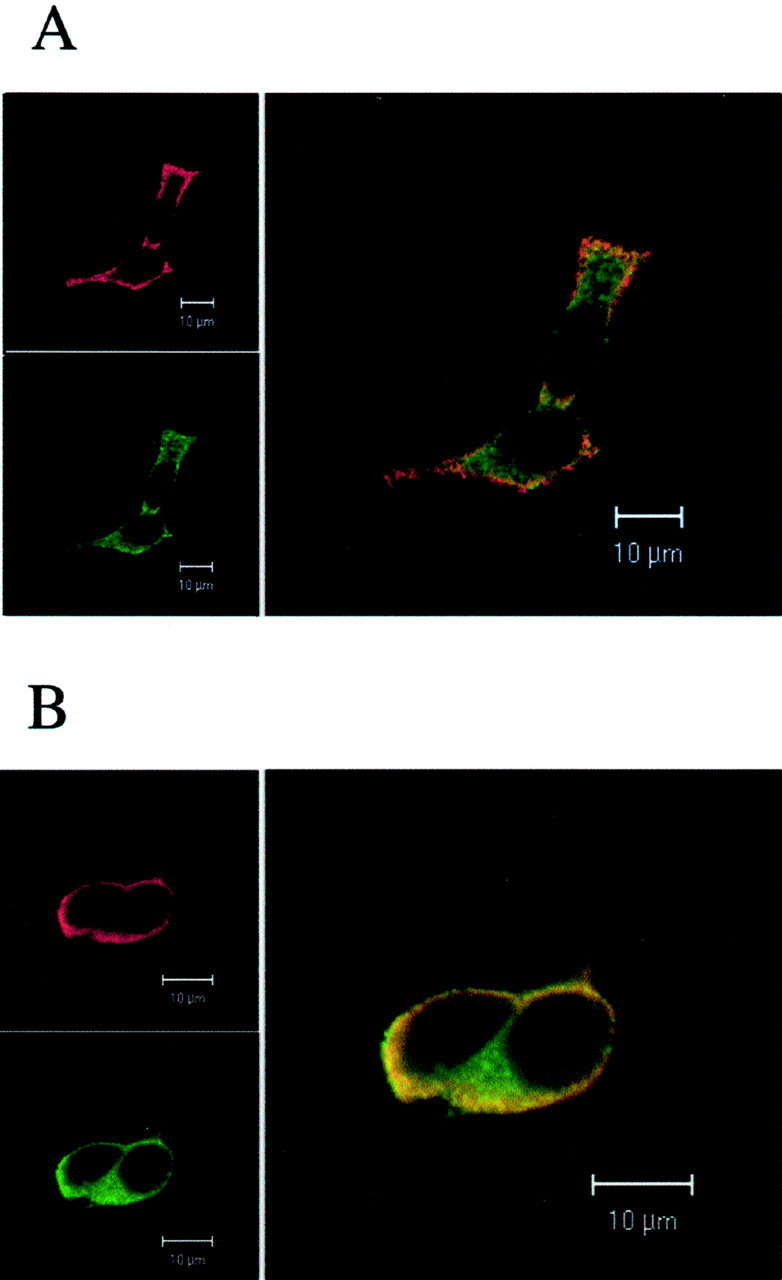
Carbachol stimulation causes microtubule depolymerization and translocation of tubulin to PIP2-enriched membrane regions of SK-N-SH-neuroblastoma cells. Cells were untreated (A) or treated with 1 mm carbachol for 2 min (B) before fixation, followed by FITC labeling of tubulin and Texas Red labeling of PIP2, as described. Carbachol-induced concentration of tubulin in the PIP2-enriched membrane and submembrane areas of the cells (B) is apparent. Tubulin–PIP2 colocalization appears inyellow. Representative images of cells obtained in one of three independent experiments with similar results are shown.
Microtubule depolymerization inhibits phosphoinositide hydrolysis in SK-N-SH cells
Exogenous tubulin regulates PLCβ1signaling when added to membranes from engineered Sf9 or SK-N-SH neuroblastoma cells (Popova and Rasenick, 2000). To test whether endogenous tubulin affected phosphoinositide hydrolysis, SK-N-SH cells were pretreated with colchicine before analysis of inositol phosphate production. Colchicine is a well known pharmacologic agent that binds to microtubules and causes microtubule depolymerization (Wilson and Jordan, 1994). Colchicine also activates tubulin GTPase in the absence of polymerization (David-Pfeuty et al., 1979; Andreu and Timasheff, 1981). Colchicine would be expected to increase the cellular concentration of tubulin–GDP dimers, which do not activate Gαq.
Endogenous phosphoinositide pools of SK-N-SH cells were prelabeled withmyo-[3H]inositol (Popova and Dubocovich, 1995), and carbachol-induced inositol phosphate generation was studied in colchicine-treated or control cells (Fig.8). Confocal immunofluorescence microscopy demonstrated significant microtubule depolymerization in colchicine-pretreated cells (Fig. 8A). Retraction of cellular projections and change in cell shape were also observed. Colocalization of tubulin and PIP2 in regions close to the membrane was also seen.
Fig. 8.
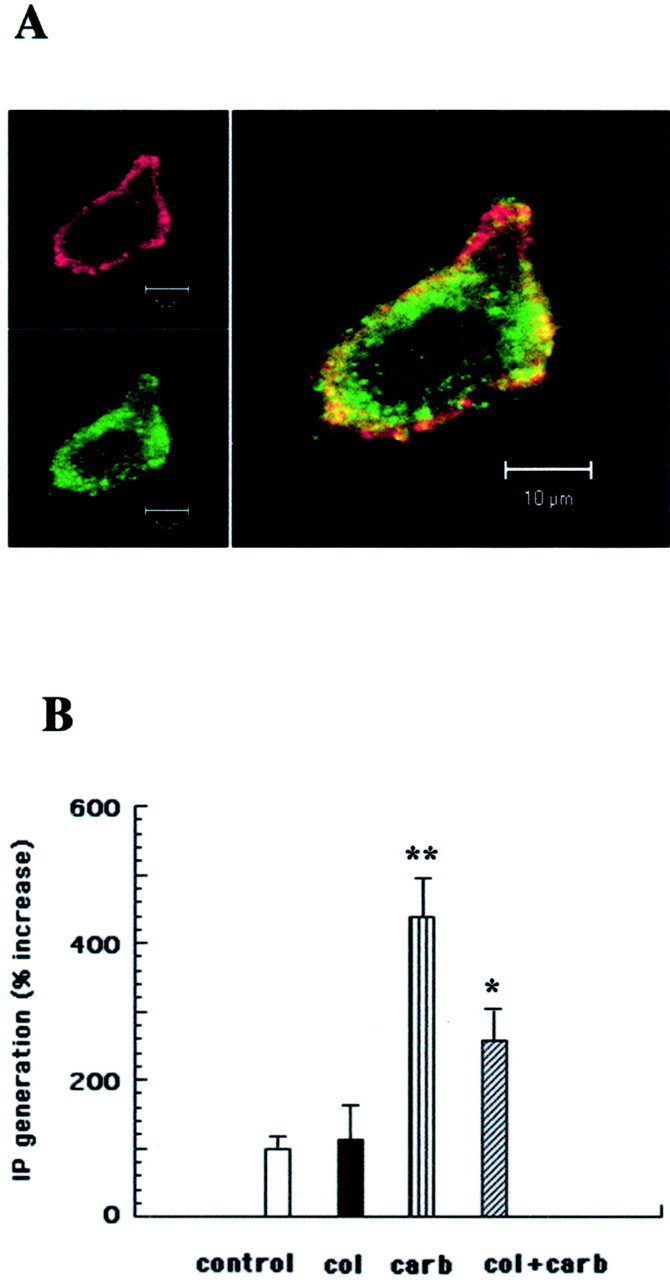
Colchicine-evoked microtubule depolymerization inhibits PLCβ signaling in SK-N-SH neuroblastoma cells.A, Confocal immunofluorescence image of an SK-N-SH cell treated for 15 min with 33 μm colchicine as described in Materials and Methods. Microtubule depolymerization as well as colocalization (yellow) of tubulin (green) with PIP2(red) is demonstrated. B,Myo-[3H]inositol-prelabeled SK-N-SH cells were treated for 15 min with 33 μm colchicine (col). Carbachol (carb; 10 μm) was added, the samples were incubated for 30 min at 37°C, and the total inositol phosphate production was measured as described. **Significantly different from control cells (p < 0.01); *significantly different from carbachol-treated cells (p < 0.05).
Colchicine treatment of SK-N-SH cells inhibited carbachol-stimulated inositol phosphate generation by 40% (Fig. 8B). Colchicine treatment did not affect the basal PLC activity of SK-N-SH cells. When present in the incubation medium, colchicine did not increase the association of purified tubulin with SK-N-SH membranes, suggesting that the increase in membrane-associated tubulin was attributable to the increase in tubulin dimer concentration.
Colchicine pretreatment did not affect the interaction of tubulin with Gαq or PLCβ1. When Sf9 membranes containing these proteins were pretreated for 15 min with colchicine (10 μm), the membrane association of 1 μmtubulin–[32P]AAGTP, induced by carbachol or PIP2, was unaltered. Carbachol (100 μm) and guanosine 5′-(β,γ-imido)triphosphate (10 μm) activation of PLCβ1 were also unaffected when Sf9 membranes were pretreated with colchicine (2.71 ± 0.2 nmol · min−1 · mg of protein−1 and 2.79 ± 0.6 nmol · min−1 · mg of protein−1 before and after colchicine, respectively).
DISCUSSION
The purpose of this study was to determine whether and how PIP2 contributes to the regulation of PLCβ1 signaling by tubulin. Although PIP2 is the preferred substrate for PLCβ1, it also binds to tubulin and shortens microtubules in vitro (Popova et al., 1997). Furthermore, low (nanomolar) concentrations of tubulin activate, whereas high (micromolar) concentrations inhibit, PLCβ1(Popova et al., 1997). Tubulin binding to Gαq, followed by transactivation of Gαq attributable to the transfer of GTP from tubulin, appears responsible for the activation phase (Popova and Rasenick, 2000). The mechanism by which tubulin inhibits PLCβ1 had not been revealed, but it appeared to involve PIP2.
A previous study (Popova et al., 1997) speculated that, at high tubulin concentrations, association of PIP2 with tubulin might render PIP2 unavailable to PLCβ1 decreasing PLCβ1activity. However, this previous study left open another possibility, which is that the binding of PIP2 might also affect the GTP-binding or -hydrolyzing properties of tubulin (Davis et al., 1994) and might render tubulin unable to transactivate Gαq (Popova et al., 1997). Data in Figures 1 and 2 showed that although PIP2 interacted with tubulin in a specific manner, it did not affect either GTP binding or GTP hydrolysis by tubulin.
The present results also demonstrate that, although PIP2 had no direct effect on Gαq transactivation by tubulin, it supported the membrane association of tubulin in both Sf9 cells, which ectopically express recombinant muscarinic receptors, Gαq, and PLCβ1, and SK-N-SH neuroblastoma cells, which normally contain these proteins (Figs. 3, 4). Colocalization of tubulin and PIP2along the plasma membrane of SK-N-SH neuroblastoma cells was also observed (Fig. 7). These results are concordant with the idea that PIP2-enriched regions of the membrane might be sites for tubulin association. Examples of such regions are lipid rafts enriched in sphingolipids and cholesterol, which sequester certain proteins but exclude others. They are considered platforms for initiation of signal transduction processes, membrane trafficking, and molecular sorting. PIP2 is present in these rafts (Laux et al., 2000). It has been shown recently that in differentiated rat cerebellar granule cells, glycerophospholipids represent 45–75% of the constituents of sphingolipid-enriched membrane domains, of which PIP2 is ∼3% (Prinetti et al., 2001). Because the protein content of these domains is ∼0.1–2.8% (Prinetti et al., 2001), protein/PIP2 ratios ranging between 1:0.8 and 1:13.5 are estimated. These values are concordant with the tubulin/PIP2 ratios used in the present study. Lipid-anchored tubulin within detergent-resistant and glycolipid-enriched plasma membrane domains has also been demonstrated (Palestini et al., 2000). Thus, specific binding of tubulin to the minor membrane lipid PIP2 might facilitate tubulin targeting to such specific membrane locations.
Membrane- or phospholipid-associated tubulin has been reported (Bhattacharyya and Wolff, 1976; Klausner et al., 1981; Kumar et al., 1981; Reaven and Azhar, 1981; Regula et al., 1986; Caron and Berlin, 1987). It appeared that this “membrane” tubulin was similar to the soluble form (Bhattacharyya and Wolff, 1976; Stephens, 1977). The recently discovered microtubule depolymerization and translocation of tubulin from the cytosol to the membrane in response to receptor stimulation showed one mechanism for tubulin targeting to the membrane (Popova and Rasenick, 2000; Ciruela and McIlhinney, 2001; this study). The finding that tubulin is posttranslationally palmitoylated (Caron, 1997; Zambito and Wolff, 1997) supports this observation, because this reversible and agonist-regulated lipid modification has been shown to facilitate association of Gα subunits with membranes (for review, see Casey, 1995; Dunphy and Linder, 1998). However, it has also been suggested that palmitoylation may be insufficient for protein targeting to the detergent-resistant membrane rafts (Melkonian et al., 1999). Additional lipid modifications or binding to additional membrane proteins or lipids may be required (Melkonian et al., 1999). Both myristoylation and palmitoylation of Gαi may be necessary for its association with liposomes and partitioning into rafts (Moffett et al., 2000). Thus, palmitate and the binding of PIP2 might similarly cooperate to anchor tubulin dimers to specific signaling domains of the plasma membrane.
A number of studies have shown PIP2-assisted membrane attachment of regulatory cytosolic proteins. PLC isozymes, phospholipase D, GTPases, guanine nucleotide exchange factors, GTPase-activating proteins, the vesicle-associated GTPase dynamin, and protein kinases interact with PIP2 at their pleckstrin homology (PH) domains (Musacchio et al., 1993; Shaw, 1993,1996; Gibson et al., 1994; Hodgkin et al., 1999). The binding of PIP2 assists the targeting of these proteins to the membrane and facilitates their coupling with membrane-associated signaling molecules. Binding with high affinity to both the activated receptor and phosphoinositides was proposed to provide a multipoint attachment of β-arrestin and arrestin 3 to the plasma membrane (Gaidarov et al., 1999). Tubulin might enjoy a similar attachment.
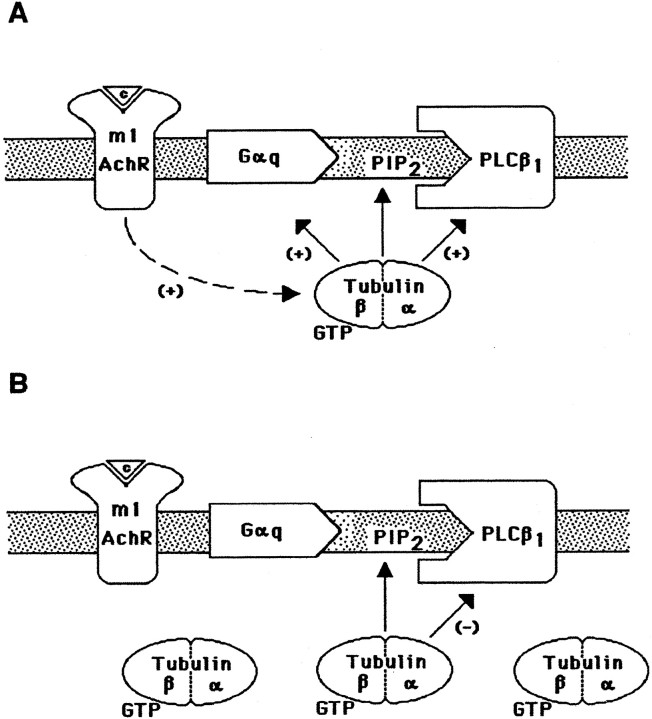
Thus, in areas proximal to the plasma membrane, PIP2 could support the receptor-evoked membrane attachment of tubulin-GTP (Popova et al., 1997; Popova and Rasenick, 2000). The subsequent involvement of tubulin in a complex with Gαq and PLCβ1 might stabilize their active conformation and potentiate PLCβ1 activation (Fig. 9A). The scenario might be quite different at high local tubulin concentrations. At high tubulin concentrations, the binding of tubulin to PIP2-rich sites of the plasma membrane proceeds in a receptor-independent manner, leading to direct association of tubulin with PLCβ1 and subsequent enzyme inhibition (Fig. 9B). Consistent with this hypothesis is the observation that high concentrations of PIP2decrease the interaction of tubulin with Gαq and high concentrations of tubulin decrease the activity of PLCβ1. Tubulin–PIP2–PLCβ1complexes should be unable to interact with receptor-activated Gαq. This notion is supported by the observation that pretreatment of SK-N-SH cells with the microtubule-depolymerizing agent colchicine decreased PLCβ1 activation.
Fig. 9.
Mechanism of tubulin regulation of PLCβ1 activity. A, Initial association of tubulin with the membrane: activation of Gq and PLCβ1. It is hypothesized that m1 muscarinic receptor stimulation triggers association of tubulin with the plasma membrane, resulting in subsequent regulation of PLCβ1 signaling. The binding of tubulin to PIP2 at the membrane supports the membrane association of tubulin and, perhaps, the formation of the active tubulin–Gαq–PLCβ1 complex. B, Increased association of tubulin with membranes inhibits PLCβ1. At high local concentrations of tubulin, receptor-independent interaction of tubulin with PLCβ1through PIP2 renders the enzyme inaccessible for receptor-activated Gαq, leading to PLCβ1 inhibition. The physiological relevance of dual regulation of PLCβ1by tubulin is supported by the observation that PLCβ1activation increases intracellular Ca2+concentration, which in turn causes microtubule depolymerization. Feedback inhibition of PLCβ1 at elevated concentrations of tubulin dimers is suggested. m1 AchR, m1Muscarinic acetylcholine receptor.
These regulatory mechanisms presuppose an agonist-modulated change in localized tubulin dimer concentration. Initially, hormone- or neurotransmitter-mediated activation of PLC would increase local Ca2+ concentrations, which, in turn, would cause microtubule depolymerization in this region of the cell. The resulting increase in tubulin dimer (Weisenberg, 1972; Serrano et al., 1986) might then provide a feedback inhibition of PLCβ1. Rapid increase in membrane-associated tubulin after carbachol treatment of cells has been demonstrated (Fig.7; Popova and Rasenick, 2000). Furthermore, Gαs and Gαi have been shown to bind tubulin and activate GTPase. This destroys the GTP cap on microtubules (Roychowdhury et al., 1999) and perhaps increases local tubulin dimer concentration in response to agonist activation of G-protein-coupled receptors. The increased association of tubulin–GDP with the membrane and subsequent inhibition of PLCβ1 after colchicine treatment are consistent with such hypotheses (Fig. 8).
The site(s) on tubulin for specific binding of PIP2 is not yet identified. PH domains on a number of signaling molecules, including G-protein-coupled receptor kinases (GRKs), have been implicated in interacting with PIP2 and G-protein βγ subunits (Musacchio et al., 1993; Shaw, 1993, 1996; Gibson et al., 1994), but not all of them bind these ligands (Davis and Bennett, 1994). Furthermore, the G-protein-coupled receptor kinase GRK5 does not possess a PH domain and does not bind Gβγ (Pitcher et al., 1996). However, GRK5 contains regions rich in basic amino acids within both its N and C termini (Pitcher et al., 1996), and these regions might represent lipid-binding domains (Kunapuli et al., 1994; Casey, 1995; Pronin et al., 1998). Although tubulin does not have a typical PH domain, it contains regions rich in basic amino acids that might be involved in the binding of PIP2. However, because other negatively charged phospholipids fail to affect tubulin polymerization, the interaction of PIP2 with tubulin appears to be specific and not solely electrostatic.
The findings described in this paper demonstrate that the specific interaction of tubulin with the integral membrane lipid and PLCβ1 substrate PIP2defines its membrane association and involvement in Gαq-mediated signaling. This reversible association might represent a highly localized phenomenon, whereby tubulin could temporarily attach to specific membrane domains for the purpose of directing G-protein-mediated signaling. This type of focal signaling, requiring local changes in calcium and microtubules, represents a continuum between G-protein signaling and the cytoskeleton.
Footnotes
This study was supported by National Institutes of Health Grants MH 39595 and AG 15482 (M.M.R.) and Council for Tobacco Research Grant 4089 (M.M.R.). We thank Dr. S. G. Rhee for providing us with PLCβ1 baculovirus and PLCβ1 antibody and Dr. J. Garrison for the Gαq baculovirus. We also thank Drs. G. Luthin, D. Manning, W. Dunn, M. Gnegy, and E. Ross for the generous gifts of material.
Correspondence should be addressed to Mark M. Rasenick, Department of Physiology and Biophysics, University of Illinois at Chicago, College of Medicine, 835 South Wolcott Avenue, m/c 901, Chicago, IL 60612-7342. E-mail raz@uic.edu.
REFERENCES
- 1.Andreu JM. Hydrophobic interactions of tubulin. Ann NY Acad Sci. 1986;466:626–630. doi: 10.1111/j.1749-6632.1986.tb38438.x. [DOI] [PubMed] [Google Scholar]
- 2.Andreu JM, Timasheff SN. The ligand- and microtubule assembly-induced GTPase activity of purified calf brain tubulin. Arch Biochem Biophys. 1981;211:151–157. doi: 10.1016/0003-9861(81)90440-9. [DOI] [PubMed] [Google Scholar]
- 3.Banno Y, Nakashima T, Kumada T, Ebisawa K, Nonomura Y, Nozawa Y. Effects of gelsolin on human platelet cytosolic phosphoinositide-phospholipase C isozymes. J Biol Chem. 1992;267:6488–6494. [PubMed] [Google Scholar]
- 4.Bhattacharyya B, Wolff J. Polymerisation of membrane tubulin. Nature. 1976;264:576–577. doi: 10.1038/264576a0. [DOI] [PubMed] [Google Scholar]
- 5.Boguslavsky V, Rebecchi M, Morris AJ, Jhon D-Y, Rhee SG, McLaughlin S. Effect of monolayer surface pressure on the activities of phosphoinositide-specific phospholipase C-β1, -γ1, and -δ1. Biochemistry. 1994;33:3032–3037. doi: 10.1021/bi00176a036. [DOI] [PubMed] [Google Scholar]
- 6.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 7.Carlier MF, Pantaloni D. Kinetic analysis of guanosine 5′-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry. 1981;20:1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- 8.Caron JM. Posttranslational modification of tubulin by palmitoylation: I. In vivo and cell-free studies. Mol Biol Cell. 1997;8:621–636. doi: 10.1091/mbc.8.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron JM, Berlin RD. Dynamic interactions between microtubules and artificial membranes. Biochemistry. 1987;26:3681–3688. doi: 10.1021/bi00386a063. [DOI] [PubMed] [Google Scholar]
- 10.Casey PJ. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- 11.Ciruela F, McIlhinney RA. Metabotropic glutamate receptor type 1α and tubulin assemble into dynamic interacting complexes. J Neurochem. 2001;76:750–757. doi: 10.1046/j.1471-4159.2001.00099.x. [DOI] [PubMed] [Google Scholar]
- 12.Cote M, Payet MD, Gallo-Payet N. Association of αs subunit of the Gs protein with microfilaments and microtubules: implication during adrenocorticotropin stimulation in rat adrenal glomerulosa cells. Endocrinology. 1997a;138:69–78. doi: 10.1210/endo.138.1.4860. [DOI] [PubMed] [Google Scholar]
- 13.Cote M, Payet MD, Dufour MN, Guillon G, Gallo-Payet N. Association of the G protein αq/α11 subunit with cytoskeleton in adrenal glomerulosa cells: role in receptor-effector coupling. Endocrinology. 1997b;138:3299–3307. doi: 10.1210/endo.138.8.5319. [DOI] [PubMed] [Google Scholar]
- 14.David-Pfeuty T, Simon C, Pantaloni D. Effect of antimitotic drugs on tubulin GTPase activity and self-assembly. J Biol Chem. 1979;254:11696–11702. [PubMed] [Google Scholar]
- 15.Davis A, Sage CR, Dougherty CA, Farrell KW. Microtubule dynamics modulated by guanosine triphosphate hydrolysis activity of beta-tubulin. Science. 1994;264:839–842. doi: 10.1126/science.8171338. [DOI] [PubMed] [Google Scholar]
- 16.Davis LH, Bennett V. Identification of two regions of βG spectrin that bind to distinct sites in brain membranes. J Biol Chem. 1994;269:4409–4416. [PubMed] [Google Scholar]
- 17.Dunphy JT, Linder ME. Signalling functions of protein palmitoylation. Biochim Biophys Acta. 1998;1436:245–261. doi: 10.1016/s0005-2760(98)00130-1. [DOI] [PubMed] [Google Scholar]
- 18.Ferguson KM, Lemmon MA, Schlessinger J, Sigler PB. Structure of the high affinity complex of inositol trisphosphate with a phospholipase C pleckstrin homology domain. Cell. 1995;83:1037–1046. doi: 10.1016/0092-8674(95)90219-8. [DOI] [PubMed] [Google Scholar]
- 19.Fisher SK, Heacock AM. A putative M3 muscarinic cholinergic receptor of high molecular weight couples to phosphoinositide hydrolysis in human SK-N-SH neuroblastoma cells. J Neurochem. 1988;50:984–987. doi: 10.1111/j.1471-4159.1988.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 20.Fukami K, Matsuoka K, Nakanishi O, Yamakawa A, Kawai S, Takenawa T. Antibody to phosphatidylinositol 4,5-bisphosphate inhibits oncogene-induced mitogenesis. Proc Natl Acad Sci USA. 1988;85:9057–9061. doi: 10.1073/pnas.85.23.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaidarov I, Krupnick JG, Falck JR, Benovic JL, Keen JH. Arrestin function in G protein-coupled receptor endocytosis requires phosphoinositide binding. EMBO J. 1999;18:871–881. doi: 10.1093/emboj/18.4.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson TJ, Hyvonen M, Birney E, Musacchio A, Saraste M. PH domain: the first anniversary. Trends Biochem Sci. 1994;19:349–353. doi: 10.1016/0968-0004(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 23.Glaven JA, Zheng Y, Wu WJ, Cerione RA. Phosphatidylinositol 4,5-bisphosphate provides an alternative to guanine nucleotide exchange factors by stimulating the dissociation of GDP from Cdc42Hs. J Biol Chem. 1996;271:23815–23819. doi: 10.1074/jbc.271.39.23815. [DOI] [PubMed] [Google Scholar]
- 24.Goldschmidt-Clermont PJ, Machesky LM, Baldassare JJ, Pollard TD. The actin-binding protein profilin binds to PIP2 and inhibits its hydrolysis by phospholipase C. Science. 1990;247:1575–1578. doi: 10.1126/science.2157283. [DOI] [PubMed] [Google Scholar]
- 25.Goldschmidt-Clermont PJ, Kim JW, Machesky LM, Rhee SG, Pollard TD. Regulation of phospholipase Cγ1 by profilin and tyrosine phosphorylation. Science. 1991;251:1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- 26.Graber SG, Figler RA, Garrison JC. Expression and purification of functional G protein α subunits using a baculovirus expression system. J Biol Chem. 1992;267:1271–1278. [PubMed] [Google Scholar]
- 27.Hodgkin MN, Masson MR, Powner D, Sagib KM, Ponting CP, Wakelam MJO. Phospholipase D regulation and localisation is dependent upon a phosphatidylinositol 4,5-biphosphate-specific PH domain. Curr Biol. 1999;17:43–46. doi: 10.1016/s0960-9822(99)00264-x. [DOI] [PubMed] [Google Scholar]
- 28.Ibarrondo J, Joubert D, Dufour MN, Cohen-Solal A, Homburger V, Jard S, Guillon G. Close association of the α subunits of Gq and G11 G proteins with actin filaments in WRK1 cells: relation to G protein-mediated phospholipase C activation. Proc Natl Acad Sci USA. 1995;92:8413–8417. doi: 10.1073/pnas.92.18.8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kavran JM, Klein DE, Lee A, Falasca M, Isakoff SJ, Skolnik EY, Lemmon M A. Specificity and promiscuity in phosphoinositide binding by pleckstrin homology domains. J Biol Chem. 1998;273:30497–30508. doi: 10.1074/jbc.273.46.30497. [DOI] [PubMed] [Google Scholar]
- 30.Klausner RD, Kumar N, Weinstein JN, Blumental R, Flavin M. Interaction of tubulin with phospholipid vesicles. I. Association with vesicles at the phase transition. J Biol Chem. 1981;256:5879–5885. [PubMed] [Google Scholar]
- 31.Kumar N, Klausner RD, Weinstein JN, Blumenthal R, Flavin M. Interaction of tubulin with phospholipid vesicles. II. Physical changes of the protein. J Biol Chem. 1981;256:5886–5889. [PubMed] [Google Scholar]
- 32.Kunapuli P, Gurevich VV, Benovic JL. Phospholipid-stimulated autophosphorylation activates the G protein-coupled receptor kinase GRK5. J Biol Chem. 1994;269:10209–10212. [PubMed] [Google Scholar]
- 33.Laux T, Fukami K, Thelen M, Golub T, Frey D, Caroni P. GAP43, MARCKS, CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, regulate cell cortex actin dynamics through a common mechanism. J Cell Biol. 2000;149:11455–11471. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 35.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 36.Musacchio A, Gibson T, Rice P, Thompson J, Saraste M. The PH domain: a common piece in the structural patchwork of signalling proteins. Trends Biochem Sci. 1993;18:343–348. doi: 10.1016/0968-0004(93)90071-t. [DOI] [PubMed] [Google Scholar]
- 37.Palestini P, Pitto M, Tedeschi G, Ferraretto A, Parenti M, Brunner J, Masserini M. Tubulin anchoring to glycolipid-enriched, detergent-resistant domains of the neuronal plasma membrane. J Biol Chem. 2000;275:9978–9985. doi: 10.1074/jbc.275.14.9978. [DOI] [PubMed] [Google Scholar]
- 38.Parker EM, Kameyama K, Higashijima T, Ross EM. Reconstitutively active G protein-coupled receptors purified from baculovirus-infected insect cells. J Biol Chem. 1991;266:519–527. [PubMed] [Google Scholar]
- 39.Pitcher JA, Fredericks ZL, Stone WC, Premont RT, Stoffel RH, Koch WJ, Lefkowitz RJ. Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies. J Biol Chem. 1996;271:24907–24913. doi: 10.1074/jbc.271.40.24907. [DOI] [PubMed] [Google Scholar]
- 40.Popova J, Rasenick MM. Muscarinic receptor activation promotes the membrane association of tubulin for the regulation of Gq-mediated phospholipase Cβ1 signaling. J Neurosci. 2000;20:2774–2782. doi: 10.1523/JNEUROSCI.20-08-02774.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popova J, Johnson GL, Rasenick MM. Chimeric Gαs/Gαi2 proteins define domains on Gαs that interact with tubulin for β-adrenergic activation of adenylyl cyclase. J Biol Chem. 1994;269:21748–21754. [PubMed] [Google Scholar]
- 42.Popova JS, Dubocovich ML. Melatonin receptor-mediated stimulation of phosphoinositide breakdown in chick brain slices. J Neurochem. 1995;64:130–138. doi: 10.1046/j.1471-4159.1995.64010130.x. [DOI] [PubMed] [Google Scholar]
- 43.Popova JS, Garrison JC, Rhee SG, Rasenick MM. Tubulin, Gq, and phosphatidylinositol 4,5-bisphosphate interact to regulate phospholipase Cβ1 signaling. J Biol Chem. 1997;272:6760–6765. doi: 10.1074/jbc.272.10.6760. [DOI] [PubMed] [Google Scholar]
- 44.Prinetti A, Chigorno V, Prioni S, Loberto N, Marano N, Tettamanti G, Sonnino S. Changes in the lipid turnover, composition, and organization, as sphingolipid-enriched membrane domains, in rat cerebellar granule cells developing in vitro. J Biol Chem. 2001;276:21136–21145. doi: 10.1074/jbc.M010666200. [DOI] [PubMed] [Google Scholar]
- 45.Pronin AN, Carman CV, Benovic JL. Structure-function analysis of G protein-coupled receptor kinase-5. Role of the carboxyl terminus in kinase regulation. J Biol Chem. 1998;273:31510–31518. doi: 10.1074/jbc.273.47.31510. [DOI] [PubMed] [Google Scholar]
- 46.Rasenick MM, Wang N. Exchange of guanine nucleotides between tubulin and GTP-binding proteins that regulate adenylate cyclase: cytoskeletal modification of neuronal signal transduction. J Neurochem. 1988;51:300–311. doi: 10.1111/j.1471-4159.1988.tb04870.x. [DOI] [PubMed] [Google Scholar]
- 47.Rasenick MM, Talluri M, Dunn WJ., III Photoaffinity guanosine 5′-triphosphate analogs as a tool for the study of GTP-binding proteins. Methods Enzymol. 1994;237:100–110. doi: 10.1016/s0076-6879(94)37055-9. [DOI] [PubMed] [Google Scholar]
- 48.Ravindra R, Kunapuli SP, Forman LJ, Nagele RG, Foster KA, Patel SA. Effect of transient overexpression of Gqα on soluble and polymerized tubulin pools in GH3 and AtT-20 cells. J Cell Biochem. 1996;61:392–401. doi: 10.1002/(SICI)1097-4644(19960601)61:3%3C392::AID-JCB6%3E3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 49.Reaven E, Azhar S. Effect of various hepatic membrane fractions on microtubule assembly-with special emphasis on the role of membrane phospholipids. J Cell Biol. 1981;89:300–308. doi: 10.1083/jcb.89.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regula CS, Sager PR, Berlin RD. Membrane tubulin. Ann NY Acad Sci. 1986;466:832–842. doi: 10.1111/j.1749-6632.1986.tb38466.x. [DOI] [PubMed] [Google Scholar]
- 51.Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 52.Roychowdhury S, Rasenick MM. Tubulin-G protein association stabilizes GTP binding and activates GTPase: cytoskeletal participation in neuronal signal transduction. Biochemistry. 1994;33:9800–9805. doi: 10.1021/bi00198a052. [DOI] [PubMed] [Google Scholar]
- 53.Roychowdhury S, Panda D, Wilson L, Rasenick MM. G protein α subunits activate tubulin GTPase and modulate microtubule polymerization dynamics. J Biol Chem. 1999;274:13485–13490. doi: 10.1074/jbc.274.19.13485. [DOI] [PubMed] [Google Scholar]
- 54.Serrano L, Valencia A, Caballero R, Avila J. Localization of the high affinity calcium-binding site on tubulin molecule. J Biol Chem. 1986;261:7076–7081. [PubMed] [Google Scholar]
- 55.Shaw G. Identification of novel pleckstrin homology (PH) domains provides a hypothesis for PH domain function. Biochem Biophys Res Commun. 1993;195:1145–1151. doi: 10.1006/bbrc.1993.2164. [DOI] [PubMed] [Google Scholar]
- 56.Shaw G. The pleckstrin homology domain: an intriguing multifunctional protein module. Bioessays. 1996;18:35–46. doi: 10.1002/bies.950180109. [DOI] [PubMed] [Google Scholar]
- 57.Shelanski M, Gaskin F, Cantor C. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA. 1973;70:765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steed PM, Nagar S, Wennogle LP. Phospholipase D regulation by a physical interaction with the actin-binding protein gelsolin. Biochemistry. 1996;35:5229–5237. doi: 10.1021/bi952370j. [DOI] [PubMed] [Google Scholar]
- 59.Stephens RE. Major membrane protein differences in cilia and flagella: evidence for a membrane-associated tubulin. Biochemistry. 1977;16:2047–2058. doi: 10.1021/bi00629a001. [DOI] [PubMed] [Google Scholar]
- 60.Sun H-Q, Lin K-M, Yin HL. Gelsolin modulates phospholipase C activity in vivo through phospholipid binding. J Cell Biol. 1997;138:811–820. doi: 10.1083/jcb.138.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terui T, Kahn RA, Randazzo PA. Effects of acid phospholipids on nucleotide exchange properties of ADP-ribosylation factor 1. Evidence for specific interaction with phosphatidylinositol 4,5-bisphosphate. J Biol Chem. 1994;269:28130–28135. [PubMed] [Google Scholar]
- 62.Wang N, Rasenick MM. Tubulin-G protein interactions involve microtubule polymerization domains. Biochemistry. 1991;30:10957–10965. doi: 10.1021/bi00109a021. [DOI] [PubMed] [Google Scholar]
- 63.Wang N, Yan K, Rasenick MM. Tubulin binds specifically to the signal-transducing proteins, Gsα and Giα1. J Biol Chem. 1990;265:1239–1242. [PubMed] [Google Scholar]
- 64.Weisenberg RC. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972;177:1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- 65.Wilson L, Jordan MA. Pharmacological probes of microtubule function. In: Hyams JS, Lloyd CW, editors. Microtubules. Wiley-Liss; New York: 1994. pp. 59–83. [Google Scholar]
- 66.Yan K, Greene E, Belga F, Rasenick MM. Synaptic membrane G proteins are complexed with tubulin in situ. J Neurochem. 1996;66:1489–1495. doi: 10.1046/j.1471-4159.1996.66041489.x. [DOI] [PubMed] [Google Scholar]
- 67.Yan K, Popova JS, Moss A, Shah B, Rasenick MM. Tubulin stimulates adenylyl cyclase activity in C6 glioma cells by bypassing the β-adrenergic receptor: a potential mechanism of G protein activation. J Neurochem. 2001;76:182–190. doi: 10.1046/j.1471-4159.2001.00013.x. [DOI] [PubMed] [Google Scholar]
- 68.Zambito AM, Wolff J. Palmitoylation of tubulin. Biochem Biophys Res Commun. 1997;239:650–654. doi: 10.1006/bbrc.1997.7525. [DOI] [PubMed] [Google Scholar]