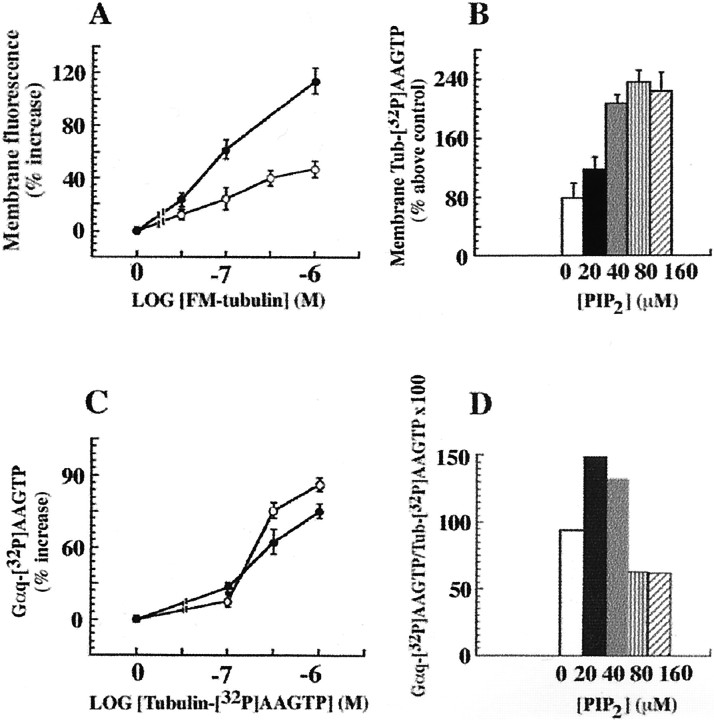
Fig. 4.
PIP2 does not increase Gαq transactivation by tubulin. A, The effects of carbachol and PIP2 on the membrane association of tubulin are additive. Recruitment of tubulin to the membrane was studied using increasing concentrations of FM-tubulin. Membranes from Sf9 cells expressing m1 muscarinic receptors, Gαq, and PLCβ1 were incubated with carbachol and FM-tubulin (at the indicated concentrations), with or without PIP2. SDS-PAGE (50 μg of membrane protein in each lane) was followed by measurement of the fluorescence of membrane-associated tubulin with a fluorescence imaging system (Storm 840; Molecular Dynamics). The results represent one of three similar experiments performed in triplicate. Open circles, Membranes treated with 1 mm carbachol; filled circles, membranes treated with 1 mm carbachol and 30 μmPIP2. B, PIP2-assisted recruitment of tubulin–[32P]AAGTP to the membrane is concentration-dependent. Membranes from Sf9 cells containing m1 muscarinic receptors, Gαq, and PLCβ1 were incubated with 1 mm carbachol, 1 μmtubulin–[32P]AAGTP, and increasing concentrations of PIP2, as described in Figure 1. After SDS-PAGE (50 μg of membrane protein in each lane)32P-labeled protein bands were measured by phosphorimage analysis. One of three identical experiments done in triplicate with similar results is shown. C, Carbachol-evoked Gαq transactivation by tubulin is not affected by PIP2. The experiments were done as described in A, except that tubulin–[32P]AAGTP was used. Proteins were resolved by SDS-PAGE (50 μg of membrane protein in each lane), and the radioactivity of the Gαq bands ([32P]AAGTP was transferred from tubulin) was measured by phosphorimage analysis (Storm 840; Molecular Dynamics). One of five independent experiments done in triplicate with similar results is shown. Open circles, Membranes treated with 1 mm carbachol;filled circles, membranes treated with 1 mmcarbachol and 30 μm PIP2. D, The increased membrane association caused by PIP2 was not linked to Gαq transactivation. The percent ratios of [32P]AAGTP-labeled Gαq and tubulin–[32P]AAGTP at the various PIP2 concentrations are derived from the experiment described in B. One of three identical experiments done in triplicate with similar results is shown. Control values forB and D represent the amount of tubulin associated with the plasma membrane in the absence of carbachol or PIP2.