Abstract
Purpose:
Retinopathy of prematurity (ROP) is widely regarded worldwide as a major cause of childhood blindness, however until recently the disease has not been recognized in most of the African continent. As a result of changing economic conditions, there is growing evidence that the population at risk for ROP in Africa is increasing. This report aims to summarize the published literature on ROP from Africa.
Methods:
We performed a systematic literature review of the English and French online literature databases by applying a general search strategy initially on May 1, 2017 with repeat inquiry on May 20, 2018. Search phrases included multiple variants of terms including “ROP”, “retinopathy of prematurity”, in conjunction with each of the individual 54 recognized sovereign African states.
Findings:
25 individual studies from 6 African nations were identified: South Africa (10), Egypt (7), Nigeria (4), with the nations of Sudan, Rwanda, and Kenya each having one respective study. 2 countries (South Africa and Kenya) have developed national ROP policies for primary and secondary prevention.
Summary:
Review of the published literature suggests that ROP is emerging in Africa, however there are published data from 6 / 54 (11%) African nations. Blindness from ROP is often preventable with appropriate primary and secondary prevention. This report provides compelling evidence that these efforts should be undertaken to implement and evaluate regionally appropriate ROP prevention programs in a growing number of African countries.
Introduction
The World Health Organization’s Vision 2020 program identified retinopathy of prematurity (ROP) as a leading cause of childhood blindness, particularly low- and middle-income countries (LMIC).1 More than 184,700 infants born prematurely worldwide in 2010 were estimated to have developed ROP of any stage, 20,000 of which later became blind or severely visually impaired.2 Infancy-acquired blindness has large implications from a societal and economic standpoint. Much of this burden however may largely be prevented through early screening and prompt treatment of vision-threating disease. LMIC are experiencing an increasing epidemic of ROP as more premature babies survive due to improved neonatal care.3 Under the implementation of the United Nation’s Millennium Development Goals, there was a 53% reduction in under-five child mortality from 1990 to 2015, nearly all of which is due to reduced mortality in the first year of life.4 From the neonatal mortality perspective, this is encouraging; however, since only an estimated 40% of neonatal intensive care units (NICUs) in LMIC settings have established ROP screening programs5, this foreshadows the emerging epidemic of childhood blindness in LMIC.
ROP is a disease that did not exist one hundred years ago. It emerged as health systems improve and neonatal mortality reduces. In LMIC, accurate estimates of the incidence of ROP in preterm infants are often lacking, and this is particularly true in Africa. With an estimated population more than a billion, Africa is the world’s second most populous continent after Asia.6 More than 60% of the world’s pre-term births occur in South Asia and Sub-Saharan Africa (SSA).7 Nigeria, the most populous country in SSA has the 3rd highest rate of prematurity in the world.7 A decade ago, ROP was not a leading cause of blindness in SSA, where diseases such as avitaminosis and measles are the primary contributors of preventable blindness, however this is changing.8–9 As a result, ROP has been largely neglected by several blindness prevention programs in Africa.6
Some of the earliest regional ROP studies conducted in Africa concluded there were no reports of ROP8–9, and until recently there were limited data regarding incidence of ROP in Africa. However, in the last decade there are an increasing number of published reports demonstrating that in certain African nations ROP is emerging. Accurate determination of ROP prevalence is essential for strategic public health planning and further health policy considerations.6 ROP screening guidelines exist for only 2 countries in Africa (South Africa and Kenya), yet this report will show that there are published data on ROP from 6 African nations. In order to advocate for the development of screening guidelines and the implementation of screening programs, this systematic review aims to address the knowledge gap that exists regarding the changing epidemiology of ROP in the African continent.
Materials and Methods
Search Strategy and Study Selection Criteria
Preferred Reporting Items for Systematic Reviews and Meta- Analyses (PRISMA) guidelines were followed throughout the search and data extraction process. Multiple databases including Web of Sciences, PubMed/EMBASE, Medline, SCOPUS, African Journal Online, Index Medicus for Eastern Mediterranean Region were searched initially on May 1, 2017 with repeat inquiry on May 20, 2018. A general search strategy was applied using MeSH terms “retinopathy of prematurity”, “retrolental fibroplasia”, or “ROP” in conjunction with MeSH terms “Africa” and each of the respective individual 54 recognized sovereign states. Additionally, French literature databases were searched using terms “Rétinopathie du premature” and “Fibroplasie rétrolentale” to encompass any additional results. Of interest were primary studies relating to the epidemiology of ROP in the neonatal period. Article duplications, meta-analyses, blindness school studies, and case reports were excluded from the final analysis. Article titles and abstracts were assessed for relevancy. Eligible studies were obtained for full-text screening. Snowball searching of reference lists was used to identify further studies of interest. No restrictions were placed on the date of study or date of article publication. Institutional Review Board approval was not required by the University of Illinois, Chicago for this review article.
Data extraction and study quality
Articles which met the selection inclusion criteria were reviewed in a full-text manner. The following data was extracted from each study: basic demographical information of each study and their respective participants, including the first author’s name, date of the study, sample size, province or country of study, the study’s inclusion criteria for screening in terms of guidelines for birthweight (BW) and gestational age (GA). Additional data that was collected included the determined ROP incidence and/or prevalence of the study as well as the mean birthweight and gestational age of the studied neonates.
Results
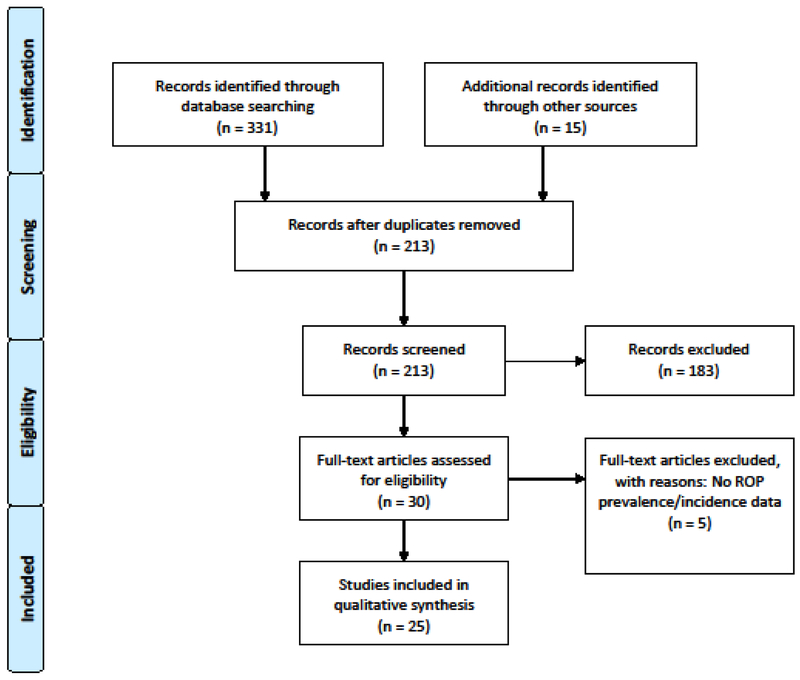
The study flowchart is shown in Figure 1, and the findings are summarized in Table 1. 25 individual studies from 6 African nations were identified with the plurality of studies emanating from South Africa (10), followed by Egypt (7), Nigeria (4), and the nations of Sudan, Rwanda, and Kenya each having one respective study. All included papers were cross-sectional studies. No additional relevant publications were found in corresponding French literature databases. The sample size of the studies was between 18 and 981 cases.
Figure 1.
Flow chart of literature review to identify eligible studies.
Table 1.
Rates of ROP Across African Nations
| Study | Publish Year | Study Year | Inclusion Criteria | Prevalence | Treatable ROP | GA (weeks) | BW (grams) |
|---|---|---|---|---|---|---|---|
| Egypt | |||||||
| El-Mekaweyy35 | 2011 | No comment | <32 weeks and <1500g or requiring O2 incubator | 226/981 (23%) | No comment | <32 n=925 (94.3%) | <1500g n=424 (43.3%) |
| Abel-Hakeem11 | 2012 | January 2009-December352010 | <32 weeks or <1500g or if exposed to oxygen therapy >7 days | 33/172 (19.2%) | 6 Patients | 33.02 +/−1.72 | 1510 +/−245 |
| Hadi37 | 2013 | January 2010-January 2012 | <32 weeks and <1250g | 52/152 (34.4%) | 15 Patients | 31.02 +/− 2.13 | 1329 +/− 280 |
| Bedda14 | 2014 | June 2012-November 2013 | <33 weeks with <1500g | 73/223 (33.74%) | 26 Patients | 31.09 +/−1.76 | 1222.62 +/−236.66 |
| Nassar13 | 2016 | January 2010-March 2011 | <32 weeks and <1500g | 19/52 (36.5%) | 5 Patients | 31.3 +/−2.8 | 1234.6 +/−221.1 |
| Bassiouny36 | 2017 | March 2013-March 2015 | <37 weeks | 237/402 (59%) | 11 Patients | 31.5 +/− 2.3 | 1514 +/− 391 |
| Ali12 | 2017 | June 2014-May 2015 | <37 weeks or <2500g | 75/108 (69.4%) | 11 Patients | 32; 29 | 1460; 1200 |
| Kenya | |||||||
| Wanjala15 | 2015 | November 2003-April 2004 | <1750g and <35 weeks | 20/120 (16.7%) | 1 Patient | 30 | 1375 |
| Onyango44 | 2018 | January 2010-December 2015 | <32 weeks or <1500g | 43/103 (41.7%) | 9 Patients | 29.9 +/− 2.2 | 1280.1 +/− 333.0 |
| Nigeria | |||||||
| Baiyeroju-Agbeja17 | 1998 | January 1995-June 1995 | <1500g or <31 weeks | 1/18 (5.5%) | None | 26–31 | 870–1500 |
| Ademola-Popoola45 | 2013 | July 2007-October 2007 | <35 weeks and <1900g | 23/29 (79%) | No Comment | 30.97 +/− 2.32 | 1988 +/− 332 |
| Adio18 | 2014 | January 2012-October 2012 | <32 weeks and <1500g | 25/53 (47.2%) | 1 Patient | 28.98 +/− 1.38 | 1411 +/− 128g |
| Fajolu19 | 2015 | November 2011-May 2014 | <32 weeks and <1500g | 12/80 (15%) | 6 Patients | 28.9 +/− 1.7 | 1231 +/− 268 |
| Rwanda | |||||||
| Uwizhiwe20 | 2016 | September 2015-March 2016 | <2500g, <37 wekks | 22/148 (14.9%) | No comment | 32.6 | 1588 |
| South Africa | |||||||
| Straker23 | 1991 | March 1988-March 1989 | <34 weeks<1500g | 27/141 (19.2%) | No Comment | 26–34 | 1021 |
| Kirsten43 | 1995 | July 1986-August 1987 | <1500g and ventilated | 40/127 (31.5%) | No Comment | 29.9 +/−2.14 | 1184.8 +/− 176.7 |
| Delport31 | 2002 | February 1999-December 1999 | <1500g | 23/94 (24.5%) | 4 Patients | 34 | 1200 |
| Mayet46 | 2006 | July 2001-December 2003 | <32 weeks <1500g | 84/514 (16.3%) | 8 Patients | 35.1 +/− 3.7 | With ROP (1093.7 +/− 173.2) Without ROP (1215.6g +/− 174.6) |
| Van Der Merwe38 | 2013 | January 2009-December 2010 | <28 weeks or <1000g | 75/356 (21.8%) | CSROP 19; laser therapy 6 | 28.3 +/− 1.7 | 949.3 |
| Keraan39 | 2016 | November 2012-May 2013 | <31 weeks or <1251 | 40/135 (29.6%) | 8 Patients | 30.1 +/− 1.9 | 1056 +/− 172 |
| Visser40 | 2016 | March 2009-February 2014 | <32 weeks <1500g | 369/1104 (33.4%) | 27 patients | 28 weeks | 930g |
| Jacoby41 | 2016 | January 2009-December 2014 | <32 weeks <1500g | 2010–13.6%; 2011 25.8%; 2012–30.9%; 2013: 31.8%; 2014: 25.0% | 2010: 0%; 2011: 2.5%; 2012: 2.6%; 2013: 1.5%; 2014: 1.0% | No Comment | No Comment |
| Dadoo42 | 2016 | January 2013-December 2013 | <1500g | 38/147 (25.8%) | 4 Patients | 29 (+/−2.74) | |
| Du Bruyn24 | 2018 | January 2008-December 2015 | GA 32 weeks or BW <1500g | 2008: 4.65% 2011: 8.76 2015: 2.36% | Treatable ROP (28.5 weeks) Non-Treatable ROP (29.25weeks) | Treatable ROP (1081g) Nontreatable ROP (1195g) | |
| Sudan | |||||||
| Omer25 | 2014 | January 2012-January 2013 | <34 weeks | 34/93 (37%) | 12 Patients | 32–34 | <1500g (57.6%) |
Country Specific Data (in alphabetical order)
Egypt
Egypt is the third-most populous country in the African continent with an estimated the number of live births around 1.88 million per year, and an estimated 136,000 preterm births.10 At the present time, no formal screening criteria for ROP has been published for Egypt. Seven studies have been published from Egypt with ROP incidence ranging from 19.2 – 69.4%.11–12 In 2012 Hakeem published a study of 172 infants within a two-year prospective report found ROP in 19.2% of studied infants (GA <32 weeks and BW <1500g or infants BW >1500g who were exposed to oxygen therapy more than seven days).11 Two studies in Alexandria, Egypt by Nassar (2016) and Bedda (2014) reported a ROP prevalence of 36.5% (GA <32 weeks and BW <1500g) and 33.7% (GA <33 weeks and BW <1500g) respectively.13–14 In 2017 Ali et al. published a retrospective cohort study reporting an incidence of 69.4% (GA <37 weeks and BW < 2500g).12 The latter study confirmed cases of ROP occurring outside of UK or AAP criteria for BW and GA: 26 patients had GA >32 weeks and 30 patients had BW >1500g.12
Kenya
Kenya has approximately 1.5 million live births each year with 188,100 infants born prematurely each year.10 One study was identified conducted by Wanjala et al during 6-month timeframe between November 2003-April 2004 at two of the largest public hospital and referral centers in Nairobi.15 Inclusion screening criteria consisted of GA of 35 weeks or less and BW 1750g or less. At the end of the follow-up period, 16.7% in a cohort of 120 babies screened were identified to have ROP, only one baby (0.8%) had threshold ROP.15 In 2018, Kenya developed national ROP screening guidelines, making it only the second country in Africa to do so. In 2018, Kenya developed national ROP screening guidelines, making it only the second country in Africa to do so. The guidelines closely follow the SA screening guidelines published in 2013, recommending screening all newborns with BW <1501g, newborns with GA <32 weeks, and high-risk neonates with BW 1501g-2000g.16
Nigeria
Nigeria is the most populous country in Africa and has the third highest number of annual preterm births globally with roughly 773,600 out of 6.3 million live births annually.10 The earliest ROP study in the region was published by Baiyeroju et al in 1995 in a 18 subject prospective study, identifying only one baby with ROP.17 The authors identified severe equipment failures and out of the 18 subjects studied, 12 died before the study was concluded.17 Since the initial study, strides have been made in neonatal care in Nigeria. Adio et al. found a prevalence of ROP in 47.2% of screened infants (GA <32 weeks and BW <1500g), but none of the identified cases progressed to severe blinding disease.18 Fajolu et al identified a prevalence of ROP in 15% of patients and 7.5% of screened infants required treatment in a study population of GA <32 weeks and BW <1500g.19 All the infants were referred to a private facility for treatment, but only one family could afford treatment.19 Nigeria has no public health insurance system so except for the small (wealthy) proportion of the population able to afford private insurance, all neonatal care services are paid out of pocket.
Rwanda
Rwanda has an estimated 437,500 live births, with roughly 41,600 babies preterm.10 Uwizihiwe et al reported a prevalence of ROP of 14.9% at one district hospital between the years of 2015–2016.20 The authors speculate the lower prevalence figure may be related to wider inclusion criteria (GA <37-weeks, BW <2500g) as the majority detected ROP were in infants <1500g.20 Additionally, the authors noted a particularly high dropout rate from the study, only 148 babies were screened out of the 294 infants originally enrolled.20
South Africa
South Africa has over 1.06 million live births each year, 84,800 of which are premature.10 The public health sector treats approximately 80% of the population in South Africa and over the last few years several policies have been implemented to reduce neonatal and infant mortality21 97% of deliveries are attended by skilled medical providers.22
The first reports of ROP in Africa were documented by Straker et al in 1991 showing a reported prevalence of 19.2% in studied cohort of GA <34 weeks and BW <1500g23. Since then publications have emerged from Cape Town, Johannesburg, Pretoria and Tygerberg. South Africa has made instrumental measures to address ROP, namely the establishment of a national screening protocol in 2013. The encouraging effects of the screening guideline implementation in SA were documented in an 8-year follow up report conducted by Du Bryun. The study stated after the screening protocol’s establishment, there were more timely referrals for the screening of premature babies a net reduction in the number of children requiring treatment secondary was appreciated. Treatable ROP decreased from 8.75% in 2011 to 3.98% in 2012 and 2.36% in 201524.
Sudan
Sudan annually has 1.4 million live births with roughly 184,000 preterm births.10 A prospective hospital-based study conducted by Omar et al in 2012–2013 demonstrated a prevalence of 37% in the 92 infants studied (GA <34 weeks).25 Of those, 12 (35.5%) were treated with laser therapy.25
Discussion
There are several key findings from this study. First, there are published data from 6 African nations. Second, reviewing the rate of ROP publications over time suggests that the disease is emerging across the continent. Prior to the year 2000, only two countries (South Africa and Nigeria) had published ROP incidence data, and some of those early papers suggested ROP was not present26. Out of the 25 publications we identified, 15 were published since 2014. Third, despite the evidence of an emerging epidemic of ROP in at least 7 African nations, only 2 have established national screening guidelines to facilitate implementation of effective primary and secondary prevention programs.
There is published evidence of ROP in Egypt, Kenya, Nigeria, Rwanda, South Africa and Sudan. Prior work has identified associated economic indicators that predict emergence of ROP.2 While the majority of the 54 African nations may indeed not yet have the developed neonatal care services to have a large population at risk for ROP, there are a number of populous countries with comparable health systems to those with published data where it is likely ROP may be emerging, such as Ethiopia, Democratic Republic of Congo, and Tanzania. These data suggest that countries with similar economic and health care infrastructure should consider implementing pilot ROP screening programs to determine whether the disease may be emerging in those countries. Allocation of resources to ROP screening where there is no evidence of ROP incidence is an interesting epidemiologic chicken vs. egg paradox. Without allocating resources to look for the disease, it may be hard to justify that those resources are being well spent.
In this study, we used publication of ROP incidence data as a research tool to identify regions with emerging ROP epidemics. Using this methodology, the incidence of ROP appears to be increasing (from close to zero in 2000) across parts of the African continent. While avoidable causes of blindness secondary to measles and vitamin A deficiency continue to remain among the leading contributors of childhood blindness in Africa, the relative prevalence of ROP-related blindness is clearly growing in Africa27–28. This is unsurprising and follows the pattern of ROP in LMIC in other regions.3 As neonatal care and mortality continues to improve throughout the continent, there will be a predictable growing need for ROP care, or a predictable emergence of a population of blind children. Further work is needed to evaluate whether there may be other cost-effective and predictive methods of identifying regions where ROP screening should be implemented without the resources typically required for large population based epidemiologic studies.
ROP screening guidelines must be regionally relevant, since birthweight and gestational age cutoffs that work in the US and UK do not often work in LMIC due to differences in primary prevention.29–30 Moreover, in the US and the UK, ROP screening guidelines are based primarily on birthweight and gestational age, both of which are independently predictive of ROP. However, in many LMIC settings, the estimation of gestational age may be unreliable. Delport et al bring to attention that because of resource constraints in developing nations (e.g. ultrasound instrumentation) and lack of routine perinatal appointments and poor patient follow up, gestational age of neonates is difficult to accurately assess. In their study, an accurate gestational age was obtainable in only about 10% of cases.31 Most pregnant mothers do not know their gestation and gestational age assessment is not accurate28. Clinical methods to determine the gestational age in a newborn infant may not be accurate as the Ballard score, for example, has 2-week variances.32 With the absence of an accurate gestational age, an infant’s birth weight remains the most reliable clinical parameter indicative of prematurity. These concerns were reflected in the recommendations for screening in SA and modified accordingly.33
South Africa and Kenya are the only 2 countries in Africa to have their own national screening protocols. The ROP guidelines for South Africa published in 2013 detail that all very-low-birth weight babies <1 500 g or 32 weeks GA should be screened for ROP.30 Screening is repeated until retinal vascularization has reached a stage where the risk of a serious adverse outcome is considered minimal.33 The 2013 guidelines recommend screening all VLBW babies at 4 – 6 weeks chronological age or 31 – 33 weeks corrected GA, whichever comes last.33 The presence of a regular screening program is important to fundamental recognition of ROP, namely raising awareness of risk factors such as unmonitored oxygen therapy and the importance of screening to physicians as well as the parents. The South African screening program is an example of what is urgently required in other developing nations in Africa in order to limit the ocular morbidity secondary to ROP. A report following implementation of the SA screening protocol demonstrated a decrease in the treatable ROP numbers at one large SA hospital.24 The authors state that the implementation of screening guidelines led to greater awareness among healthcare providers, resulting in more timeous referrals of premature babies for ROP screening and treatment. Rates of treatable of ROP were reported at 4.65% in 2008 to 8.76% in 2011, then 3.98% in 2012 and 2.36% in 2015.24 While financial constraints may limit equipment and staffing, raising awareness and shared urgency through a common screening guideline and protocol may come to bridge many of those gaps and standardize neonatal care (e.g. oxygen administration and monitoring) in the prevention of ROP.
ROP care models may look different in the African context than they do in the US/UK. Emerging innovative technologies may help to compensate for the many resource constraints required for safe and effective ROP screening. Due to a limited number of trained ophthalmologists available to screen and manage babies for ROP, there may be a stronger rationale to consider telemedicine for ROP screening, which has worked effectively in the Indian context. Telemedicine systems reduce the human resource utilization but often require high expenditure of capital resources for the acquisition of expensive fundus cameras.34 Mobile phone fundus photography or other Health approaches may also be useful in ROP screening, particularly in regions where more expensive cameras are not available, but will require careful prospective validation
Conclusion
Africa is a world within a continent with widely varied health care infrastructure both within and between countries. There are countries where allocation of resources for ROP does not make sense in 2018. However, as the continent makes steady progress towards the fulfillment of the millennium development goals, these data suggest the epidemiology is likely to continue to change in the coming years. The history of ROP has taught us that blindness from ROP can be prevented in the majority of cases with effective primary and secondary prevention, but it has also taught that improving neonatal without effective ROP prevention programs can lead to a rapid increase in the prevalence of childhood blindness. These data provide compelling evidence that the time is here to implement and evaluate regionally appropriate ROP prevention programs in a growing number of African countries.
Acknowledgments:
The authors would like to acknowledge Margaret Chervinko, MS (University of Illinois-Chicago) for her assistance with the data collection.
Financial support: Supported by grants P30 EY010572, K12 EY027720 from the National Institutes of Health (Bethesda, MD), and by unrestricted departmental funding from Research to Prevent Blindness (New York, NY).
Footnotes
This submission has not been previously published (or submitted) elsewhere.
None of the following authors have any proprietary interests or conflicts of interest related to this submission.
Contributor Information
Daniel Wang, Department of Ophthalmology, New York Eye and Ear Infirmary.
Roseline Duke, University of Calabar, Nigeria.
RV Paul Chan, Center for Global Health, College of Medicine University of Illinois, Chicago.
J. Peter Campbell, Department of Ophthalmology, Oregon Health & Science University.
References
- 1.World Health Organization and the International Agency for the Prevention of Blindness Joint Initiative. Vision 2020: Global Initiative for the Elimination of Avoidable Blindness: Action Plan 2006–2011. Geneva: World Health Organization, 2007. Access at: http://www.who.int/blindness/Vision2020_report.pdf [Google Scholar]
- 2.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74 Suppl 1:35–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert Clare, et al. “Retinopathy of Prematurity in Middle-Income Countries.” The Lancet. 1997;350: 12–14. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization, UNICEF, United Nations, and World Bank. Levels & Trends in Child Mortality: Report 2015: Estimates Developed by the UN Inter-Agency Group for Child Mortality Estimation. 2015.
- 5.Fielder Alistair, et al. “Impact of Retinopathy of Prematurity on Ocular Structures and Visual Functions.” Archives of Disease in Childhood - Fetal and Neonatal Edition. 2014;100:179–184. [DOI] [PubMed] [Google Scholar]
- 6.Ademola-Popoola D, Olyleye T. Retinopathy of prematurity in a developing economy with improving health care. Curr Ophthalmol Rep. 2017;5:114. [Google Scholar]
- 7.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172 [DOI] [PubMed] [Google Scholar]
- 8.O’Sullivan J, Gilbert C, Foster A. The causes of childhood blindness South Africa. S Afr Med J 1997;87(12):1691–1695. [PubMed] [Google Scholar]
- 9.Kello AB, Gilbert C. Causes of severe visual impairment and blindness in children in schools for the blind in Ethiopia. Br J Ophthalmol. 2003;87:526–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. http://www.who.int/pmnch/media/news/2012/201204_borntoosoon_countryranking.pdf.
- 11.Hakeem AH, Mohamed GB, Othman MF. Retinopathy of prematurity: a study of prevalence and risk factors. Middle East Afr J Ophthalmol. 2012;19:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ali AA, Gomaa NAS, Awadein AR, Al-Hayouti HH, Hegazy AI. Retrospective cohort study shows that the risks for retinopathy of prematurity included birth age and weight, medical conditions and treatment. Acta Paediatr. 2017;106:1919–1927. [DOI] [PubMed] [Google Scholar]
- 13.Nassar MM. Screening for retinopathy of prematurity: a report from upper Egypt. Int J Ophthalmol. 2016;9:262–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bedda AM, Al NMAE, Abd-Elhady A, Ahmad ISH. Evaluation of the treatment of retinopathy of prematurity in preterm infants in Alexandria University Hospital. Journal of the Egyptian Ophthalmological Society. 2014;107:70. [Google Scholar]
- 15.Wanjala I, Ilako D, Kariuki L. Retinopathy of Prematurity as seen in two major hospitals in Nairobi, Kenya. East Africa Journal of Ophthalmology. 2007;13:5–14. [Google Scholar]
- 16.Mwangi N, Onyango O, Gachago M, Murila F, Njambi L. National Guidelines for The Screening and Management of Retinopathy of Prematurity. Nairobi: Ministry of Health Kenya; 2018. [Google Scholar]
- 17.Baiyeroju-Agbeja A, Omokhodion SI. Screening for retinopathy of prematurity in Idadan. Nigerian J Ophthalmol. 1998;6:23–25. [Google Scholar]
- 18.Adio AO, Ugwu RO, Nwokocha CG, Eneh AU. Retinopathy of prematurity in port harcourt, Nigeria. ISRN Ophthalmol. 2014;2014:481527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fajolu I, Rotimi-Samuel A, Aribaba O, et al. Retinopathy of prematurity and associated factors in Lagos, Nigeria. Paediatrics and international child health. 2015;35:324–328. [DOI] [PubMed] [Google Scholar]
- 20.Uwizihiwe F. Prevalence and predisposing factors of retinopathy of prematurity in low birth weight preterm neonates at one district of Rwanda: a case of Muhima District Hospital, Neonatal unit (Master thesis, University of Rwanda). Kigali, Rwanda, University of Rwanda, 2016. 49pp. [Google Scholar]
- 21.Varughese S, Gilbert C, Pieper C, Cook C. Retinopathy of prematurity in South Africa: an assessment of needs, resources and requirements for screening programmes. Br J Ophthalmol. 2008;92:879–882. [DOI] [PubMed] [Google Scholar]
- 22.National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), and ICF. 2017. South Africa Demographic and Health Survey 2016: Key Indicators. Pretoria, South Africa, and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF. [Google Scholar]
- 23.Straker CA, van der Elst CW. The incidence of retinopathy of prematurity at Groote Schuur Hospital, Cape Town. S Afr Med J. 1991;80:287–288. [PubMed] [Google Scholar]
- 24.du Bruyn A, Visser L. Eight years of treatable retinopathy of prematurity in KwaZulu-Natal: Are we winning the battle? South African Ophthalmology Journal. 2017;12:8–10. [Google Scholar]
- 25.Omer IM, Hassan HA. The prevalence and risk factors of retinopathy of prematurity among preterm babies admitted to Soba Neonatal Intensive Care Unit. Sudan J Paediatr. 2014;14:17–21. [PMC free article] [PubMed] [Google Scholar]
- 26.Gilbert C, Waddel K, Wood M, Foster A. Causes of childhood blindness in East Africa: Results in 491 pupils attending 17 schools for the blind in Malawi, Kenya and Uganda, Ophthalmic Epidemiology. 1995; 2:2, 77–84. [DOI] [PubMed] [Google Scholar]
- 27.Gywali R, Bhayal BK, Adhikary R, Shrestha A, Sah RP. Retrospective Data on Causes of Childhood Vision Impairment in Eritrea. BMC Ophthalmology. 2017;17:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aghaji A, Okoye O, Bowman R. Causes and emerging trends of childhood blundness: findings from schools for the blind in Southeast Nigeria. Br J Ophthalmol, 2015;99:727–731. [DOI] [PubMed] [Google Scholar]
- 29.Başmak H, Niyaz L, Sahin A, Erol N, Gürsoy HH. Retinopathy of prematurity: screening guidelines need to be reevaluated for developing countries. Eur J Ophthalmol. 2010;20:752–755. [DOI] [PubMed] [Google Scholar]
- 30.Akcakaya AA, Yaylali SA, Erbil HH, et al. Screening for retinopathy of prematurity in a tertiary hospital in Istanbul: incidence and risk factors. J Pediatr Ophthalmol Strabismus. 2012;49:21–25. [DOI] [PubMed] [Google Scholar]
- 31.Delport SD, Swanepoel JC, Odendaal PJ, Roux P. Incidence of retinopathy of prematurity in very-low-birth-weight infants born at Kalafong Hospital, Pretoria. S Afr Med J. 2002;92:986–990. [PubMed] [Google Scholar]
- 32.Ballard JL, Khoury JC, Wedig K, Wang L, Eilers-Walsman BL, Lipp R. New Ballard Score, expanded to include extremely premature infants. J Pediatr. 1991;119:417–423. [DOI] [PubMed] [Google Scholar]
- 33.Visser L, Singh R, Young M, Lewis H, McKerrow N. Guideline for the prevention, screening and treatment of retinopathy of prematurity (ROP). S Afr Med J. 2012;103:116–125. [DOI] [PubMed] [Google Scholar]
- 34.Salcone EM, Johnston S, VanderVeen D. Review of the use of digital imaging in retinopathy of prematurity screening. Semin Ophthalmol. 2010;25:214–7. [DOI] [PubMed] [Google Scholar]
- 35.El-Mekawey H Ocular morbidity in Egyptian preterm infants discovered during screening for retinopathy of prematurity. The Medical Journal of Cairo University. 2011;79. [Google Scholar]
- 36.Bassiouny RMR, Ellakkany RS, Aboelkhair SA, Mohsen TA, Othman IS. Incidence and risk factors of retinopathy of prematurity in neonatal intensive care units: Mansoura, Egypt. Journal of the Egyptian Ophthalmological Society. 2017;110:71. [Google Scholar]
- 37.Hadi AM, Hamdy IS. Correlation between risk factors during the neonatal period and appearance of retinopathy of prematurity in preterm infants in neonatal intensive care units in Alexandria, Egypt. Clin Ophthalmol. 2013;7:831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Merwe SK, Freeman N, Bekker A, Harvey J, Smith J. Prevalence of and risk factors for retinopathy of prematurity in a cohort of preterm infants treated exclusively with non-invasive ventilation in the first week after birth. S Afr Med J. 2013;103:96–100. [DOI] [PubMed] [Google Scholar]
- 39.Keraan Q, Tinley C, Horn A, Pollock T, Steffen J, Joolay Y. Retinopathy of prematurity in a cohort of neonates at Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J. 2016;107:64–69. [DOI] [PubMed] [Google Scholar]
- 40.Visser Kift E, Freeman N, Cook C, Myer L. Retinopathy of prematurity screening criteria and workload implications at Tygerberg Children’s Hospital, South Africa: A cross-sectional study. S Afr Med J. 2016;106. [DOI] [PubMed] [Google Scholar]
- 41.Jacoby MR, Du Toit L. Screening for retinopathy of prematurity in a provincial hospital in Port Elizabeth, South Africa. S Afr Med J. 2016;106. [DOI] [PubMed] [Google Scholar]
- 42.Dadoo Z, Ballot DE. An evaluation of the screening for retinopathy of prematurity in very-low-birth-weight babies at a tertiary hospital in Johannesburg, South Africa. South African Journal of Child Health. 2016;10:79–82. [Google Scholar]
- 43.Kirsten GF, van Zyl JI, le Grange M, Ancker E, van Zyl F. The outcome at 12 months of very-low-birth-weight infants ventilated at Tygerberg Hospital. S Afr Med J. 1995;85:649–654. [PubMed] [Google Scholar]
- 44.Onyango O, Sitati S, Amolo L, Murila F, Wariua S, Nyamu G, Lango M, Patel A. Retinopathy of prematurity in Kenya: prevalence and risk factors in a hospital with advanced neonatal care. Pan Afr Med J. 2018;29:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ademola-Popoola D, Adesiyun O, Obasa TO. Screening programme for retinopathy of prematurity in Ilorin, Nigeria: a pilot study. West Afr J Med. 2013;32(4):281–5. [PubMed] [Google Scholar]
- 46.Mayet I, Cockinos C. Retinopathy of prematurity in South Africans at a tertiary hospital: a prospective study. Eye (Lond). 2006;20(1): 29–31. [DOI] [PubMed] [Google Scholar]
- 47.Kurawa MS, Mohammed I, Farouk ZL, Muhammed A. Screening for retinopathy of prematurity by practicing paediatricians and ophthalmologists in Nigeria: A survey of attitude and experience. Nigerian Journal of Basic and Clinical Sciences. 2018;15:148. [Google Scholar]