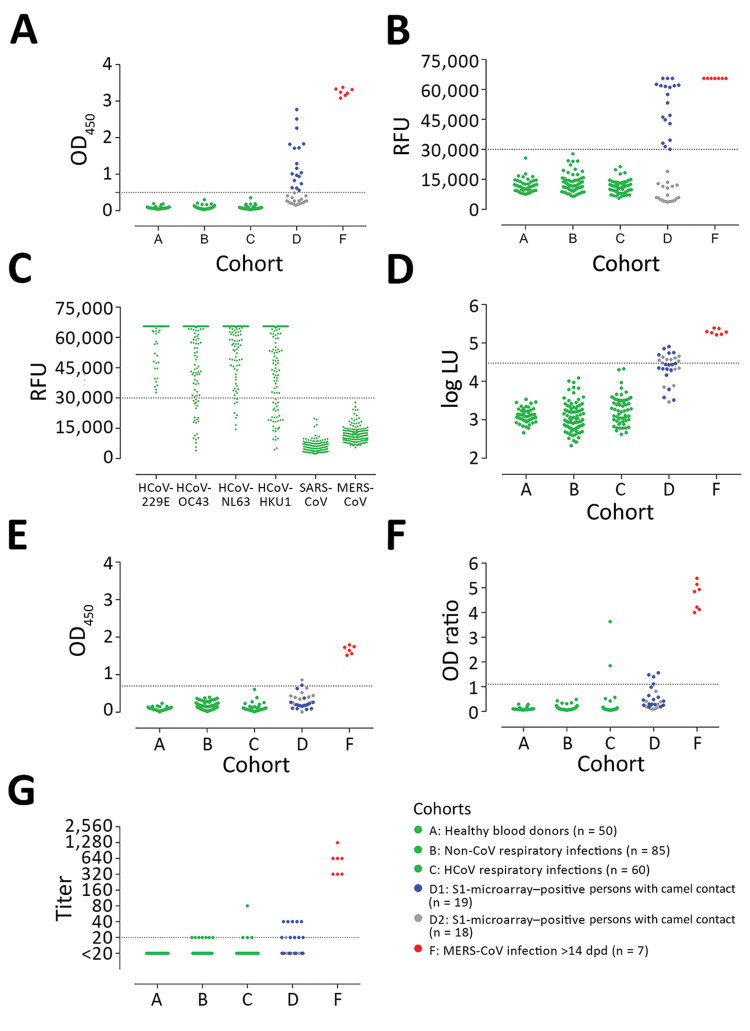
Figure 2.
MERS-CoV–specific antibody responses detected by different assay platforms. A) In-house IgG of S1 ELISA (iELISA); B) MERS-CoV S1 protein microarray; C) HCoV S1 microarray reactivity of non-MERS-CoV–infected serum samples to the S1 proteins of 6 different HCoVs; D) nucleocapsid-luciferase immunoprecipitation assay; E) IgG S2 ELISA; F) routinely used IgG S1 ELISA expressed as the ratio of optical density of sample to kit calibrator; G) plaque reduction neutralization test (PRNT), expressed as endpoint titer for 90% plaque reduction. Serum samples tested were obtained from healthy blood donors (n = 50, cohort A); patients with PCR-diagnosed respiratory infections including human coronaviruses (n = 145, cohorts B and C); S1-microarray positive (n = 18, cohort D1) and negative (n = 19, cohort D2) camel contacts; and longitudinal serum samples from 2 PCR-confirmed MERS-CoV–infected patients taken 15–228 days after diagnosis (n = 7, cohort F). Cohort E is not included because patients in this cohort were in the acute phase of infection (<14 days postdiagnosis), in which seroconversion may not have occurred. Cohorts A, B, C, and F are from the Netherlands, cohort D from Qatar. Serum samples were tested at dilutions 1:101 for ELISA and N-LIPS, 1:20 for S1 microarray, and 1:20 to 1:2,560 for PRNT. Dotted lines indicate cutoff for each assay. CoV, coronavirus; LU, luminescence units; MERS, Middle East respiratory syndrome; OD, optical density; RFU, relative fluorescence units.