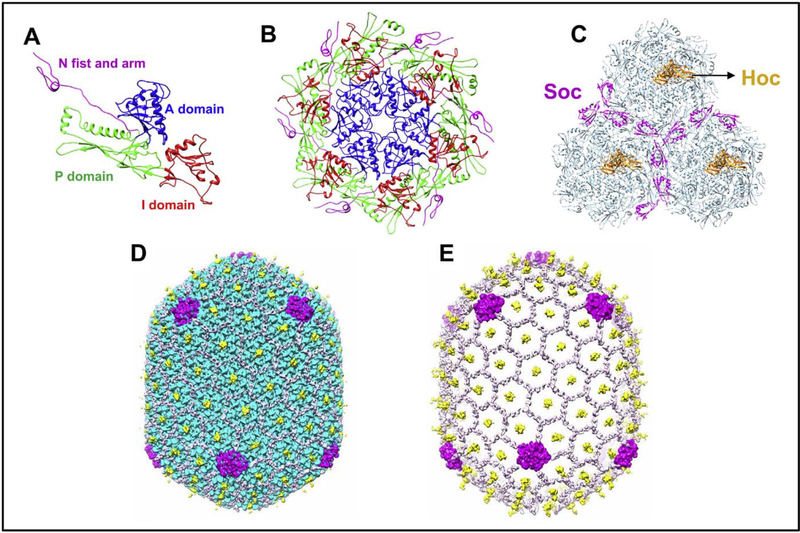
Fig. 3.
Various assembled states of the major capsid protein gp23*; monomer (A), hexameric capsomer (B), and three capsomers (C) of the hexagonal capsid lattice showing Soc subunits (magenta) bound at the interfaces of adjacent capsomers. Hoc monomers (orange) are located at the center of each capsomer. The structures are derived from the cryo-EM structure of the isometric phage T4 capsid [84]. The monomer (A) shows key domains of gp23* that associate through an intricate network of interactions to form the capsomer (B) and the capsid lattice (C). The structure is reinforced by trimeric Soc clamps at the quasi three-fold axes forming a molecular cage around the capsid (D). The capsid subunits are masked in E to depict the Soc molecular cage.