Abstract
Microglia are the resident, innate immune cells of the central nervous system (CNS) and are critical in managing CNS injuries and infections. Microglia also maintain CNS homeostasis by influencing neuronal development, viability, and function. However, aberrant microglial activity and phenotypes are associated with CNS pathology, including autism spectrum disorder (ASD). Thus, improving our knowledge of microglial regulation could provide insights into the maintenance of CNS homeostasis as well as the prevention and treatment of ASD. Control of microglial activity is in part overseen by small, lipid-derived molecules known as endogenous cannabinoids (endocannabinoids). Endocannabinoids are one component of the endocannabinoid system (ECS), which also includes the enzymes that metabolize these ligands, in addition to cannabinoid receptor 1 (CB1) and 2 (CB2). Interestingly, increased ECS signaling leads to an anti-inflammatory, neuroprotective phenotype in microglia. Here, we review the literature and propose that ECS signaling represents a largely untapped area for understanding microglial biology and its relationship to ASD, with special attention paid to issues surrounding the use of recreational cannabis (marijuana). We also discuss major questions within the field and suggest directions for future research.
Keywords: microglia, endocannabinoids, autism, neuroinflammation, neurodevelopment
Introduction
Microglia represent a self-sustaining population of cells that originates from the yolk sac and colonizes the brain in utero (Alliot et al., 1999; Ginhoux et al., 2010; Bruttger et al., 2015; Askew et al., 2017; Huang et al., 2018). Microglia are the resident immune cells of the central nervous system (CNS) and thus are the first line of defense against CNS infection and injury. For example, they phagocytize debris and pathogens as well as initiate neuroinflammatory responses through release of cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) (Yang et al., 2010; Janda et al., 2018). They also recruit natural killer cells, macrophages, and lymphocytes to sites of infection and injury (Yang et al., 2010). Moreover, microglia influence the health of their local environment through release of neurotrophic and neurotoxic factors (Nakajima et al., 2001; Srinivasan et al., 2004; Parkhurst et al., 2013).
In addition to the aforementioned roles, microglia carry out other functions essential for CNS homeostasis (Saijo and Glass, 2011; Butovsky and Weiner, 2018; Lenz and Nelson, 2018). Specifically, these cells oversee neurogenesis by both phagocytizing and directing the migration of newborn neurons (Sierra et al., 2010; Ribeiro Xavier et al., 2015). Microglia also regulate neuronal connections by engulfing excessive synaptic structures through use of the classical complement cascade (Stevens et al., 2007). Lastly, microglia modulate neuronal plasticity via release of neurotrophins such as brain-derived neurotrophic factor (BDNF) (Parkhurst et al., 2013). Early wiring of the brain requires tight control of these processes and therefore microglia critically impact CNS development (Paolicelli et al., 2011; Bialas and Stevens, 2013; Shigemoto-Mogami et al., 2014).
Due to their wide-ranging contributions to CNS homeostasis and development, microglia displaying irregular activity and/or phenotypes can lead to disorders of the CNS (Salter and Stevens, 2017; Butovsky and Weiner, 2018). In this review, we focus on microglial dysfunction as it relates to autism spectrum disorder and associated conditions. We also discuss the role of the endogenous cannabinoid system in modulating microglial involvement in these disorders.
Microglia and Autism Spectrum Disorder
The Autism and Developmental Disabilities Monitoring Network estimates the current prevalence of autism spectrum disorder (ASD) to be 1 in 59 among children in the United States (Baio et al., 2018). ASD denotes a collection of heterogeneous neurodevelopmental disorders defined by (1) repetitive, restricted behaviors and interests and (2) abnormalities in socio-communication (American Psychiatry Association, 2013). Thus, ASD is an umbrella term and it encompasses several disorders including autism, Asperger’s syndrome, pervasive developmental disorder not otherwise specified, and childhood disintegrative disorder (American Psychiatry Association, 2013). Conditions frequently comorbid with ASD include intellectual disability, attention-deficit/hyperactivity disorder, epilepsy, perturbed sleep patterns, aggression, anxiety, and altered sensory perception (Leitner, 2014; Srivastava and Schwartz, 2014; Fakhoury, 2015; Park et al., 2016; Postorino et al., 2016). These associated conditions can vary in severity and be more or less common within patient subsets. Finally, due to the lack of available therapeutics for ASD, there is a continuous, pressing need for investigation into the causes and progression of the disorder.
Given their role in CNS development and neuroinflammation, microglia are poised to influence the pathogenesis of ASD (Edmonson et al., 2016; Salter and Stevens, 2017; Lenz and Nelson, 2018). Evidence for microglial involvement in ASD comes from both post-mortem- and positron-emission tomography (PET)-imaging studies which show increased neuroinflammation and numbers of activated microglia in brains of ASD patients (Vargas et al., 2005; Morgan et al., 2010; Suzuki et al., 2013). More recently, a large-scale analysis of transcriptomic datasets from post-mortem cerebral cortex has revealed a distinct microglial signature in ASD brains (Gandal et al., 2018). This is concordant with previous observations of microglial activation-related gene enrichment in ASD brain-derived gene networks (Voineagu et al., 2011).
Altered synaptic density is observed in post-mortem ASD brain tissue (Hutsler and Zhang, 2010) and ASD mouse models (Comery et al., 1997; Tang et al., 2014; Wang et al., 2017). These alterations are presumably due to deficits in developmental synaptic pruning (Hansel, 2019). Indeed, current thinking posits that microglia can exert control over the progression of ASD through synaptic pruning dysregulation (Di Marco et al., 2016; Lenz and Nelson, 2018). This hypothesis is supported by the finding that inhibiting microglial autophagy leads to increased synaptic density and reduced sociability in mice (Kim et al., 2017). Moreover, mice with loss of microglia-enriched fractalkine receptor CX3C-chemokine receptor 1 (CX3CR1) display impaired synaptic pruning and reduced social interactions (Zhan et al., 2014). Additional support for the involvement of microglia in ASD pathogenesis comes from studies on mouse models of Rett syndrome (RTT), a syndromic form of ASD caused by mutations in the gene encoding methyl-CpG binding protein 2 (MECP2) (Lombardi et al., 2015). In one model of RTT, neuronal, but not microglial, loss of Mecp2 leads to excessive synaptic engulfment by microglia in later stages of the disease (Schafer et al., 2016). This suggests that neuronal loss of MECP2 is sufficient to induce aberrant microglial activity and it is consistent with the observation that deletion of Mecp2 using a Cx3cr1-Cre line does not produce RTT-like symptoms (Wolf et al., 2017). Furthermore, Mecp2-null microglia produce toxic levels of glutamate that damage post-synaptic structures in vitro (Maezawa and Jin, 2010). Still, due to the phenotypic and genetic heterogeneity of ASD, it remains to be seen if these findings in RTT models are representative of autism etiology in general.
Finally, children born to mothers who experience infections or autoimmune disease during their pregnancies are more likely to develop ASD (Jiang et al., 2016; Careaga et al., 2017). This phenomenon, known as maternal immune activation (MIA), has been phenocopied in rodent models (Shi et al., 2003; Patterson, 2011; Careaga et al., 2017; Salter and Stevens, 2017). While embryonic microglia may mediate the neuroinflammatory consequences of MIA (Salter and Stevens, 2017), how this underlies ASD remains unclear.
Considered together, the aforementioned findings implicate microglia as targets for the treatment of ASD. Due to its anti-inflammatory effects, the endogenous cannabinoid (endocannabinoid) system represents a promising tool for modulating microglial involvement in ASD. We next provide a brief overview of the endocannabinoid system and then summarize the evidence linking microglial-endocannabinoid signaling to ASD, with attention paid to issues surrounding the use of recreational cannabis.
The Architecture of the Endocannabinoid System
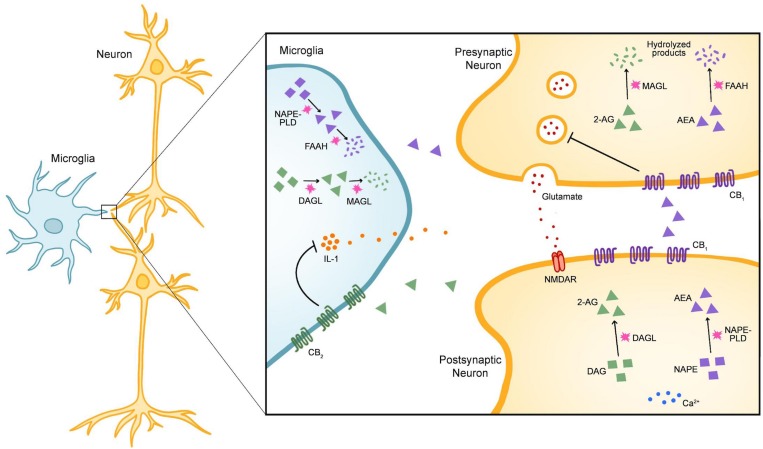
The endocannabinoid system (ECS) exerts control over microglial activity and therefore shows promise for treating CNS dysfunction (Benito et al., 2008; Stella, 2009; Lisboa et al., 2016). The ECS consists of three major components: (1) small, lipid-derived endocannabinoids (eCBs), (2) the enzymes responsible for synthesizing and degrading eCBs, and (3) the metabotropic receptors that recognize eCBs (Lutz et al., 2015). The most well-known eCBs in the brain are N-arachidonoylethanolamine (AEA or anandamide) and 2-arachidonoylglycerol (2-AG) (Lutz et al., 2015; Parsons and Hurd, 2015). In response to increased cytoplasmic calcium, 2-AG and AEA are synthesized on demand from lipid precursors by the enzymes diacylglycerol lipase (DAGL) and N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD), respectively (Alger and Kim, 2011; Lutz et al., 2015; Parsons and Hurd, 2015). The enzyme primarily responsible for degrading 2-AG is monoacylglycerol lipase (MAGL), whereas AEA is catabolized by fatty acid amide hydrolase (FAAH). In the CNS, these components are expressed in neurons, microglia, astrocytes, and oligodendrocytes (Figure 1; Stella, 2009; Lutz et al., 2015; Ilyasov et al., 2018). The two main receptors for eCBs include cannabinoid receptors 1 (CB1) and 2 (CB2), both of which are G protein-coupled (Parsons and Hurd, 2015). Finally, while CB1 is enriched in neurons, CB2 expression is primarily restricted to microglia (Stella, 2009).
FIGURE 1.
The Components of the Endogenous Cannabinoid System in Microglia and Neurons. In the central nervous system (CNS), the endogenous cannabinoids (eCBs) N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) are the most widely-recognized ligands of the endogenous cannabinoid (endocannabinoid) system (ECS). The two main receptors for eCBs are cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), both of which are G-protein coupled. Within the CNS, eCB signaling is classically understood to modulate synaptic activity. In the example given here, release of glutamate from presynaptic neurons activates N-methyl-D-aspartate receptors (NMDARs) in postsynaptic neurons. In response to increased cytoplasmic calcium, the enzyme diacylglycerol lipase (DAGL) catalyzes the synthesis of 2-AG from diacylgylcerol (DAG) and N-acyl-phosphatidylethanolamine phospholipase D (NAPE-PLD) catalyzes the synthesis of AEA from the precursor N-acylphosphatidylethanolamine (NAPE). After 2-AG and AEA are released into the synaptic cleft, they stimulate CB1 receptors on presynaptic neurons and inhibit further neurotransmitter release. 2-AG is mainly degraded by the enzyme monoacylglycerol lipase (MAGL) whereas AEA is degraded by fatty-acid amide hydrolase (FAAH). While DAGL, NAPE-PLD, MAGL, and FAAH are expressed broadly throughout the CNS, CB1 is enriched in neurons and CB2 is enriched in microglia. Stimulation of CB2 leads to a protective phenotype in microglia that is characterized by a reduction in the release of pro-inflammatory cytokines such as interleukin-1 (IL-1).
Acute consumption of Cannabis sativa (marijuana) yields wide-ranging effects on memory, cognition, appetite, and mood in both humans and rodents (Curran et al., 2016; Kendall and Yudowski, 2016). These effects result from action of the phytocannabinoid (or plant-derived cannabinoid) Δ 9-tetrahydrocannabinol (THC) on CB1 within the brain (Panagis et al., 2014; Curran et al., 2016; Kendall and Yudowski, 2016). Yet, long-term consequences of cannabis use have been poorly studied, especially with regards to microglia and their impact on neuronal circuitry. Notably, increased eCB signaling is associated with an anti-inflammatory, protective phenotype in microglia (Benito et al., 2008; Stella, 2009; Lisboa et al., 2016). For example, pharmacological inhibition of FAAH decreases microglial activation marker expression, cytokine production, and synaptic plasticity deficits, in the hippocampi of aged rats (Murphy et al., 2012). Additionally, stimulation of CB2 inhibits microglial activation and increases striatal neuron survival and motor coordination in a model of Huntington’s disease excitotoxicity (Palazuelos et al., 2009). Moreover, exposure to anti-inflammatory cytokines increases eCB production and CB2 expression in microglia (Mecha et al., 2015). These findings cast the ECS as an attractive target for influencing microglial activity (Dhopeshwarkar and Mackie, 2014; Lisboa et al., 2016; Cassano et al., 2017; Donvito et al., 2018). However, the consequences of manipulating eCB signaling on ASD risk and pathogenesis are largely uncharacterized. The increasing legality and use of cannabis currently seen throughout the world therefore requires a better understanding of eCB signaling in microglia as it relates to ASD.
Microglial-Endocannabinoid Signaling and ASD
Cannabis Use and ASD Risk
Approximately 2.5–5% of people between the ages of 15–64 years old consume cannabis, making it the most popular illicit drug in the world (Gunn et al., 2016). THC readily crosses the fetal-placental barrier (Wu et al., 2011) and is also secreted in breast milk (Perez-Reyes and Wall, 1982). As of now, there is no strong link between prenatal cannabis use and an increased risk of ASD in offspring. Prenatal exposure to cannabis does correlate with negative outcomes in child development, including growth restriction (Zuckerman et al., 1989; El Marroun et al., 2009; Gunn et al., 2016) and decreased cognitive performance (Richardson et al., 2002; Goldschmidt et al., 2004; Gunn et al., 2016). Still, few studies have been carried out on this topic and these observations are not always reproducible (Wu et al., 2011; El Marroun et al., 2018). Since rates of cannabis use in both pregnant and non-pregnant women are steadily increasing (Brown et al., 2017), there is an unmet need for clarifying the relationship between prenatal cannabis exposure and CNS development. Thus, future clinical and pre-clinical investigations should focus on elucidating the long-lasting effects of prenatal cannabis exposure and if these effects are linked to ASD pathogenesis. Such studies should be paired with efforts to determine if prenatal cannabis exposure impacts microglial synaptic pruning and thereby neurodevelopment in general. These experiments could also take place in the context of pathogen-induced MIA to better recapitulate environmental risks for ASD.
Cannabinoid Signaling as a Target for ASD Treatment
Pre-clinical evidence supporting the role of ECS signaling in ASD comes from research on rodent models of MIA and neuroinflammation. For instance, in response to the innate immunostimulant polyinosinic:polycytidylic acid [poly(I:C)], MIA-based production of IL-17 induces abnormal cortical development and ASD-like sociability deficits in mouse offspring (Choi et al., 2016). Interestingly, administration of AEA decreases IL-17 production and increases expression of the anti-inflammatory cytokine IL-10 in a mouse model of immune hypersensitivity (Jackson et al., 2014). Treatment with the innate immunostimulant lipopolysaccharide (LPS) during adolescence leads to increased AEA tone and FAAH activity in the amygdala, as well as decreased sociability, in mice (Doenni et al., 2016). These alterations are attenuated with administration of an FAAH inhibitor (Doenni et al., 2016). Still, the contribution of microglia to either the initiation or resolution of these neuroinflammatory effects is unknown and should be the focus of future endeavors.
Cannabidiol (CBD), the second major phytocannabinoid in cannabis (Atakan, 2012), has gained attention as a possible treatment for ASD (Salgado and Castellanos, 2018). Indeed, three clinical reports have recently established that CBD alleviates major symptoms associated with ASD, including seizures, sleeplessness, and anxiety, in children (Barchel et al., 2018; Aran et al., 2019; Bar-Lev Schleider et al., 2019). In addition, because CBD possess a weak affinity for CB1 and CB2, it has no psychoactive effects and may even prevent some of the harmful consequences of THC (Zuardi et al., 2012; Morales et al., 2017; Mouro et al., 2019). Lastly, a commercially available, oral CBD extract (Epidiolex) has recently gained FDA-approval for treatment of drug-resistant epilepsy (Sekar and Pack, 2019), which can be an additional burden faced by ASD patients (Sansa et al., 2011; Kokoszka et al., 2017; Long et al., 2019). Nevertheless, because exposure to other cannabinoids negatively affects the development of the adolescent brain in rats (Cha et al., 2006; Schneider and Koch, 2007; Quinn et al., 2008), parents and physicians should practice extreme caution when recommending the use of cannabinoids to treat ASD (Atakan, 2012).
Subsequent work in this field must emphasize replicating the usefulness of ECS signaling in ASD via paradigms that include larger and more diverse populations. If these results hold, it will be important to establish if abatement of ASD symptoms is due to eCB signaling in microglia. For example, CBD blocks microglial activation (Martin-Moreno et al., 2011) and neuroinflammation (Elliott et al., 2018; Maroon and Bost, 2018), both of which are linked to seizure susceptibility (Rana and Musto, 2018; Zhao et al., 2018). Consequently, it will be beneficial to establish if CBD-based reduction of epilepsy in ASD patients is reliant on microglial-based mechanisms. Utilizing mice with microglia-specific loss of ECS components in combination with ASD-relevant mouse models could shed light on this area.
Conclusion
Microglia are indispensable orchestrators of CNS development and homeostasis and are therefore likely involved in the pathogenesis of ASD. Microglial activity can be modulated by eCB signaling, which makes the ECS a potentially forceful tool in the prevention and management of CNS dysfunction. Future work must focus on detailing the mechanisms by which altered eCB signaling in microglia yields protective and detrimental effects in the CNS, particularly as it relates to the effects of chronic cannabis use. Answering these questions could provide improved therapeutics for ASD and its associated conditions.
Author Contributions
All authors wrote the manuscript. KT and DA designed the figure.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank Dr. Marissa Co for providing invaluable comments in this manuscript.
Footnotes
Funding. This work was supported by a National Institutes of Health (NIH) postdoctoral training grant (T32AI100829-06A1 to DA) and an NIH research project grant (1R01HD092093 to KS).
References
- Alger B. E., Kim J. (2011). Supply and demand for endocannabinoids. Trends Neurosci. 34 304–315. 10.1016/j.tins.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alliot F., Godin I., Pessac B. (1999). Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 117 145–152. 10.1016/s0165-3806(99)00113-3 [DOI] [PubMed] [Google Scholar]
- American Psychiatry Association (2013). Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th Edn Arlington, VA: American Psychiatry Association. [Google Scholar]
- Aran A., Cassuto H., Lubotzky A., Wattad N., Hazan E. (2019). Brief report: cannabidiol-rich cannabis in children with autism spectrum disorder and severe behavioral problems-a retrospective feasibility study. J. Autism Dev. Disord. 49 1284–1288. 10.1007/s10803-018-3808-2 [DOI] [PubMed] [Google Scholar]
- Askew K., Li K., Olmos-Alonso A., Garcia-Moreno F., Liang Y., Richardson P., et al. (2017). Coupled proliferation and apoptosis maintain the rapid turnover of microglia in the adult brain. Cell Rep. 18 391–405. 10.1016/j.celrep.2016.12.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakan Z. (2012). Cannabis, a complex plant: different compounds and different effects on individuals. Ther. Adv. Psychopharmacol. 2 241–254. 10.1177/2045125312457586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baio J., Wiggins L., Christensen D. L., Maenner M. J., Daniels J., Warren Z., et al. (2018). Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill. Summ. 67 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchel D., Stolar O., De-Haan T., Ziv-Baran T., Saban N., Fuchs D. O., et al. (2018). Oral cannabidiol use in children with autism spectrum disorder to treat related symptoms and Co-morbidities. Front. Pharmacol. 9:1521. 10.3389/fphar.2018.01521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Lev Schleider L., Mechoulam R., Saban N., Meiri G., Novack V. (2019). Real life experience of medical cannabis treatment in autism: analysis of safety and efficacy. Sci. Rep. 9:200. 10.1038/s41598-018-37570-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito C., Tolon R. M., Pazos M. R., Nunez E., Castillo A. I., Romero J. (2008). Cannabinoid CB2 receptors in human brain inflammation. Br. J. Pharmacol. 153 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialas A. R., Stevens B. (2013). TGF-beta signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat. Neurosci. 16 1773–1782. 10.1038/nn.3560 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Brown Q. L., Sarvet A. L., Shmulewitz D., Martins S. S., Wall M. M., Hasin D. S. (2017). Trends in marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA 317 207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruttger J., Karram K., Wortge S., Regen T., Marini F., Hoppmann N., et al. (2015). Genetic cell ablation reveals clusters of local self-renewing microglia in the mammalian central nervous system. Immunity 43 92–106. 10.1016/j.immuni.2015.06.012 [DOI] [PubMed] [Google Scholar]
- Butovsky O., Weiner H. L. (2018). Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19 622–635. 10.1038/s41583-018-0057-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Careaga M., Murai T., Bauman M. D. (2017). Maternal immune activation and autism spectrum disorder: from rodents to nonhuman and human primates. Biol. Psychiatry 81 391–401. 10.1016/j.biopsych.2016.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassano T., Calcagnini S., Pace L., De Marco F., Romano A., Gaetani S. (2017). Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front. Neurosci. 11:30. 10.3389/fnins.2017.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha Y. M., White A. M., Kuhn C. M., Wilson W. A., Swartzwelder H. S. (2006). Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol. Biochem. Behav. 83 448–455. 10.1016/j.pbb.2006.03.006 [DOI] [PubMed] [Google Scholar]
- Choi G. B., Yim Y. S., Wong H., Kim S., Kim H., Kim S. V., et al. (2016). The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science 351 933–939. 10.1126/science.aad0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comery T. A., Harris J. B., Willems P. J., Oostra B. A., Irwin S. A., Weiler I. J., et al. (1997). Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proc. Natl. Acad. Sci. U.S.A. 94 5401–5404. 10.1073/pnas.94.10.5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran H. V., Freeman T. P., Mokrysz C., Lewis D. A., Morgan C. J., Parsons L. H. (2016). Keep off the grass? Cannabis, cognition and addiction. Nat. Rev. Neurosci. 17 293–306. 10.1038/nrn.2016.28 [DOI] [PubMed] [Google Scholar]
- Dhopeshwarkar A., Mackie K. (2014). CB2 Cannabinoid receptors as a therapeutic target-what does the future hold? Mol. Pharmacol. 86 430–437. 10.1124/mol.114.094649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marco B., Bonaccorso C. M., Aloisi E., D’Antoni S., Catania M. V. (2016). Neuro-Inflammatory mechanisms in developmental disorders associated with intellectual disability and autism spectrum disorder: a neuro- immune perspective. CNS Neurol. Disord. Drug Targets 15 448–463. 10.2174/1871527315666160321105039 [DOI] [PubMed] [Google Scholar]
- Doenni V. M., Gray J. M., Song C. M., Patel S., Hill M. N., Pittman Q. J. (2016). Deficient adolescent social behavior following early-life inflammation is ameliorated by augmentation of anandamide signaling. Brain Behav. Immun. 58 237–247. 10.1016/j.bbi.2016.07.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donvito G., Nass S. R., Wilkerson J. L., Curry Z. A., Schurman L. D., Kinsey S. G., et al. (2018). The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology 43 52–79. 10.1038/npp.2017.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonson C. A., Ziats M. N., Rennert O. M. (2016). A non-inflammatory role for microglia in autism spectrum disorders. Front. Neurol. 7:9. 10.3389/fneur.2016.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Marroun H., Brown Q. L., Lund I. O., Coleman-Cowger V. H., Loree A. M., Chawla D., et al. (2018). An epidemiological, developmental and clinical overview of cannabis use during pregnancy. Prev. Med. 116 1–5. 10.1016/j.ypmed.2018.08.036 [DOI] [PubMed] [Google Scholar]
- El Marroun H., Tiemeier H., Steegers E. A., Jaddoe V. W., Hofman A., Verhulst F. C., et al. (2009). Intrauterine cannabis exposure affects fetal growth trajectories: the generation R study. J. Am. Acad. Child Adolesc. Psychiatry 48 1173–1181. 10.1097/CHI.0b013e3181bfa8ee [DOI] [PubMed] [Google Scholar]
- Elliott D. M., Singh N., Nagarkatti M., Nagarkatti P. S. (2018). Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 9:1782. 10.3389/fimmu.2018.01782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhoury M. (2015). Autistic spectrum disorders: a review of clinical features, theories and diagnosis. Int. J. Dev. Neurosci. 43 70–77. 10.1016/j.ijdevneu.2015.04.003 [DOI] [PubMed] [Google Scholar]
- Gandal M. J., Haney J. R., Parikshak N. N., Leppa V., Ramaswami G., Hartl C., et al. (2018). Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 359 693–697. 10.1126/science.aad6469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330 841–845. 10.1126/science.1194637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt L., Richardson G. A., Cornelius M. D., Day N. L. (2004). Prenatal marijuana and alcohol exposure and academic achievement at age 10. Neurotoxicol. Teratol. 26 521–532. 10.1016/j.ntt.2004.04.003 [DOI] [PubMed] [Google Scholar]
- Gunn J. K., Rosales C. B., Center K. E., Nunez A., Gibson S. J., Christ C., et al. (2016). Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open 6:e009986. 10.1136/bmjopen-2015-009986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel C. (2019). Deregulation of synaptic plasticity in autism. Neurosci. Lett. 688 58–61. 10.1016/j.neulet.2018.02.003 [DOI] [PubMed] [Google Scholar]
- Huang Y., Xu Z., Xiong S., Sun F., Qin G., Hu G., et al. (2018). Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat. Neurosci. 21 530–540. 10.1038/s41593-018-0090-8 [DOI] [PubMed] [Google Scholar]
- Hutsler J. J., Zhang H. (2010). Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 1309 83–94. 10.1016/j.brainres.2009.09.120 [DOI] [PubMed] [Google Scholar]
- Ilyasov A. A., Milligan C. E., Pharr E. P., Howlett A. C. (2018). The endocannabinoid system and oligodendrocytes in health and disease. Front. Neurosci. 12:733. 10.3389/fnins.2018.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. R., Nagarkatti P., Nagarkatti M. (2014). Anandamide attenuates Th-17 cell-mediated delayed-type hypersensitivity response by triggering IL-10 production and consequent microRNA induction. PLoS One 9:e93954. 10.1371/journal.pone.0093954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda E., Boi L., Carta A. R. (2018). Microglial phagocytosis and its regulation: a therapeutic target in Parkinson’s disease? Front. Mol. Neurosci. 11:144. 10.3389/fnmol.2018.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H. Y., Xu L. L., Shao L., Xia R. M., Yu Z. H., Ling Z. X., et al. (2016). Maternal infection during pregnancy and risk of autism spectrum disorders: a systematic review and meta-analysis. Brain Behav. Immun. 58 165–172. 10.1016/j.bbi.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Kendall D. A., Yudowski G. A. (2016). Cannabinoid receptors in the central nervous system: their signaling and roles in disease. Front. Cell Neurosci. 10:294. 10.3389/fncel.2016.00294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. J., Cho M. H., Shim W. H., Kim J. K., Jeon E. Y., Kim D. H., et al. (2017). Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol. Psychiatry 22 1576–1584. 10.1038/mp.2016.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoszka M. A., McGoldrick P. E., La Vega-Talbott M., Raynes H., Palmese C. A., Wolf S. M., et al. (2017). Epilepsy surgery in patients with autism. J. Neurosurg. Pediatr. 19 196–207. 10.3171/2016.7.PEDS1651 [DOI] [PubMed] [Google Scholar]
- Leitner Y. (2014). The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front. Hum. Neurosci. 8:268. 10.3389/fnhum.2014.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz K. M., Nelson L. H. (2018). Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front. Immunol. 9:698. 10.3389/fimmu.2018.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa S. F., Gomes F. V., Guimaraes F. S., Campos A. C. (2016). Microglial cells as a link between cannabinoids and the immune hypothesis of psychiatric disorders. Front. Neurol. 7:5. 10.3389/fneur.2016.00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardi L. M., Baker S. A., Zoghbi H. Y. (2015). MECP2 disorders: from the clinic to mice and back. J. Clin. Invest. 125 2914–2923. 10.1172/JCI78167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S., Zhou H., Li S., Wang T., Ma Y., Li C., et al. (2019). The clinical and genetic features of co-occurring epilepsy and autism spectrum disorder in chinese children. Front. Neurol. 10:505. 10.3389/fneur.2019.00505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B., Marsicano G., Maldonado R., Hillard C. J. (2015). The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 16 705–718. 10.1038/nrn4036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maezawa I., Jin L. W. (2010). Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci. 30 5346–5356. 10.1523/JNEUROSCI.5966-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroon J., Bost J. (2018). Review of the neurological benefits of phytocannabinoids. Surg. Neurol. Int. 9:91. 10.4103/sni.sni_45_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Moreno A. M., Reigada D., Ramirez B. G., Mechoulam R., Innamorato N., Cuadrado A., et al. (2011). Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: relevance to Alzheimer’s disease. Mol. Pharmacol. 79 964–973. 10.1124/mol.111.071290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecha M., Feliu A., Carrillo-Salinas F. J., Rueda-Zubiaurre A., Ortega-Gutierrez S., de Sola R. G., et al. (2015). Endocannabinoids drive the acquisition of an alternative phenotype in microglia. Brain Behav. Immun. 49 233–245. 10.1016/j.bbi.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Morales P., Hurst D. P., Reggio P. H. (2017). Molecular targets of the phytocannabinoids: a complex picture. Prog. Chem. Org. Nat. Prod. 103 103–131. 10.1007/978-3-319-45541-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. T., Chana G., Pardo C. A., Achim C., Semendeferi K., Buckwalter J., et al. (2010). Microglial activation and increased microglial density observed in the dorsolateral prefrontal cortex in autism. Biol. Psychiatry 68 368–376. 10.1016/j.biopsych.2010.05.024 [DOI] [PubMed] [Google Scholar]
- Mouro F. M., Miranda-Lourenco C., Sebastiao A. M., Diogenes M. J. (2019). From cannabinoids and neurosteroids to statins and the ketogenic diet: new therapeutic avenues in rett syndrome? Front. Neurosci. 13:680. 10.3389/fnins.2019.00680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy N., Cowley T. R., Blau C. W., Dempsey C. N., Noonan J., Gowran A., et al. (2012). The fatty acid amide hydrolase inhibitor URB597 exerts anti-inflammatory effects in hippocampus of aged rats and restores an age-related deficit in long-term potentiation. J. Neuroinflammation 9:79. 10.1186/1742-2094-9-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K., Honda S., Tohyama Y., Imai Y., Kohsaka S., Kurihara T. (2001). Neurotrophin secretion from cultured microglia. J. Neurosci. Res. 65 322–331. 10.1002/jnr.1157 [DOI] [PubMed] [Google Scholar]
- Palazuelos J., Aguado T., Pazos M. R., Julien B., Carrasco C., Resel E., et al. (2009). Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain 132 3152–3164. 10.1093/brain/awp239 [DOI] [PubMed] [Google Scholar]
- Panagis G., Mackey B., Vlachou S. (2014). Cannabinoid regulation of brain reward processing with an emphasis on the role of CB1 receptors: a step back into the future. Front. Psychiatry 5:92. 10.3389/fpsyt.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolicelli R. C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333 1456–1458. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]
- Park H. R., Lee J. M., Moon H. E., Lee D. S., Kim B. N., Kim J., et al. (2016). A short review on the current understanding of autism spectrum disorders. Exp. Neurobiol. 25 1–13. 10.5607/en.2016.25.1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst C. N., Yang G., Ninan I., Savas J. N., Yates J. R., III, Lafaille J. J., et al. (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155 1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. H., Hurd Y. L. (2015). Endocannabinoid signalling in reward and addiction. Nat. Rev. Neurosci. 16 579–594. 10.1038/nrn4004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P. H. (2011). Maternal infection and immune involvement in autism. Trends Mol. Med. 17 389–394. 10.1016/j.molmed.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes M., Wall M. E. (1982). Presence of delta9-tetrahydrocannabinol in human milk. N. Engl. J. Med. 307 819–820. 10.1056/nejm198209233071311 [DOI] [PubMed] [Google Scholar]
- Postorino V., Fatta L. M., Sanges V., Giovagnoli G., De Peppo L., Vicari S., et al. (2016). Intellectual disability in autism spectrum disorder: investigation of prevalence in an italian sample of children and adolescents. Res. Dev. Disabil. 48 193–201. 10.1016/j.ridd.2015.10.020 [DOI] [PubMed] [Google Scholar]
- Quinn H. R., Matsumoto I., Callaghan P. D., Long L. E., Arnold J. C., Gunasekaran N., et al. (2008). Adolescent rats find repeated Delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33 1113–1126. 10.1038/sj.npp.1301475 [DOI] [PubMed] [Google Scholar]
- Rana A., Musto A. E. (2018). The role of inflammation in the development of epilepsy. J. Neuroinflammation 15:144. 10.1186/s12974-018-1192-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro Xavier A. L., Kress B. T., Goldman S. A., Lacerda de Menezes J. R., Nedergaard M. (2015). A distinct population of microglia supports adult neurogenesis in the subventricular zone. J. Neurosci. 35 11848–11861. 10.1523/JNEUROSCI.1217-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G. A., Ryan C., Willford J., Day N. L., Goldschmidt L. (2002). Prenatal alcohol and marijuana exposure: effects on neuropsychological outcomes at 10 years. Neurotoxicol. Teratol. 24 309–320. 10.1016/s0892-0362(02)00193-9 [DOI] [PubMed] [Google Scholar]
- Saijo K., Glass C. K. (2011). Microglial cell origin and phenotypes in health and disease. Nat. Rev. Immunol. 11 775–787. 10.1038/nri3086 [DOI] [PubMed] [Google Scholar]
- Salgado C. A., Castellanos D. (2018). Autism spectrum disorder and cannabidiol: have we seen this movie before? Glob. Pediatr. Health 5:2333794X18815412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter M. W., Stevens B. (2017). Microglia emerge as central players in brain disease. Nat. Med. 23 1018–1027. 10.1038/nm.4397 [DOI] [PubMed] [Google Scholar]
- Sansa G., Carlson C., Doyle W., Weiner H. L., Bluvstein J., Barr W., et al. (2011). Medically refractory epilepsy in autism. Epilepsia 52 1071–1075. 10.1111/j.1528-1167.2011.03069.x [DOI] [PubMed] [Google Scholar]
- Schafer D. P., Heller C. T., Gunner G., Heller M., Gordon C., Hammond T., et al. (2016). Microglia contribute to circuit defects in Mecp2 null mice independent of microglia-specific loss of Mecp2 expression. eLife 5:e15224. 10.7554/eLife.15224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M., Koch M. (2007). The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur. Neuropsychopharmacol. 17 180–186. 10.1016/j.euroneuro.2006.03.009 [DOI] [PubMed] [Google Scholar]
- Sekar K., Pack A. (2019). Epidiolex as adjunct therapy for treatment of refractory epilepsy: a comprehensive review with a focus on adverse effects. F1000Res 8:234. 10.12688/f1000research.16515.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L., Fatemi S. H., Sidwell R. W., Patterson P. H. (2003). Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J. Neurosci. 23 297–302. 10.1523/jneurosci.23-01-00297.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto-Mogami Y., Hoshikawa K., Goldman J. E., Sekino Y., Sato K. (2014). Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J. Neurosci. 34 2231–2243. 10.1523/JNEUROSCI.1619-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A., Encinas J. M., Deudero J. J., Chancey J. H., Enikolopov G., Overstreet-Wadiche L. S., et al. (2010). Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7 483–495. 10.1016/j.stem.2010.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan B., Roque C. H., Hempstead B. L., Al-Ubaidi M. R., Roque R. S. (2004). Microglia-derived pronerve growth factor promotes photoreceptor cell death via p75 neurotrophin receptor. J. Biol. Chem. 279 41839–41845. 10.1074/jbc.m402872200 [DOI] [PubMed] [Google Scholar]
- Srivastava A. K., Schwartz C. E. (2014). Intellectual disability and autism spectrum disorders: causal genes and molecular mechanisms. Neurosci. Biobehav. Rev. 46(Pt 2), 161–174. 10.1016/j.neubiorev.2014.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N. (2009). Endocannabinoid signaling in microglial cells. Neuropharmacology 56(Suppl. 1), 244–253. 10.1016/j.neuropharm.2008.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens B., Allen N. J., Vazquez L. E., Howell G. R., Christopherson K. S., Nouri N., et al. (2007). The classical complement cascade mediates CNS synapse elimination. Cell 131 1164–1178. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]
- Suzuki K., Sugihara G., Ouchi Y., Nakamura K., Futatsubashi M., Takebayashi K., et al. (2013). Microglial activation in young adults with autism spectrum disorder. JAMA Psychiatry 70 49–58. [DOI] [PubMed] [Google Scholar]
- Tang G., Gudsnuk K., Kuo S. H., Cotrina M. L., Rosoklija G., Sosunov A., et al. (2014). Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron 83 1131–1143. 10.1016/j.neuron.2014.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas D. L., Nascimbene C., Krishnan C., Zimmerman A. W., Pardo C. A. (2005). Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann. Neurol. 57 67–81. 10.1002/ana.20315 [DOI] [PubMed] [Google Scholar]
- Voineagu I., Wang X., Johnston P., Lowe J. K., Tian Y., Horvath S., et al. (2011). Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature 474 380–384. 10.1038/nature10110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Li H., Takumi T., Qiu Z., Xu X., Yu X., et al. (2017). Distinct defects in spine formation or pruning in two gene duplication mouse models of autism. Neurosci. Bull. 33 143–152. 10.1007/s12264-017-0111-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y., Boura-Halfon S., Cortese N., Haimon Z., Sar Shalom H., Kuperman Y., et al. (2017). Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol. 18 665–674. 10.1038/ni.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. S., Jew C. P., Lu H. C. (2011). Lasting impacts of prenatal cannabis exposure and the role of endogenous cannabinoids in the developing brain. Future Neurol. 6 459–480. 10.2217/fnl.11.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang I., Han S. J., Kaur G., Crane C., Parsa A. T. (2010). The role of microglia in central nervous system immunity and glioma immunology. J. Clin. Neurosci. 17 6–10. 10.1016/j.jocn.2009.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y., Paolicelli R. C., Sforazzini F., Weinhard L., Bolasco G., Pagani F., et al. (2014). Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17 400–406. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]
- Zhao H., Zhu C., Huang D. (2018). Microglial activation: an important process in the onset of epilepsy. Am. J. Transl. Res. 10 2877–2889. [PMC free article] [PubMed] [Google Scholar]
- Zuardi A. W., Crippa J. A., Hallak J. E., Bhattacharyya S., Atakan Z., Martin-Santos R., et al. (2012). A critical review of the antipsychotic effects of cannabidiol: 30 years of a translational investigation. Curr. Pharm. Des. 18 5131–5140. 10.2174/138161212802884681 [DOI] [PubMed] [Google Scholar]
- Zuckerman B., Frank D. A., Hingson R., Amaro H., Levenson S. M., Kayne H., et al. (1989). Effects of maternal marijuana and cocaine use on fetal growth. N. Engl. J. Med. 320 762–768. 10.1056/nejm198903233201203 [DOI] [PubMed] [Google Scholar]