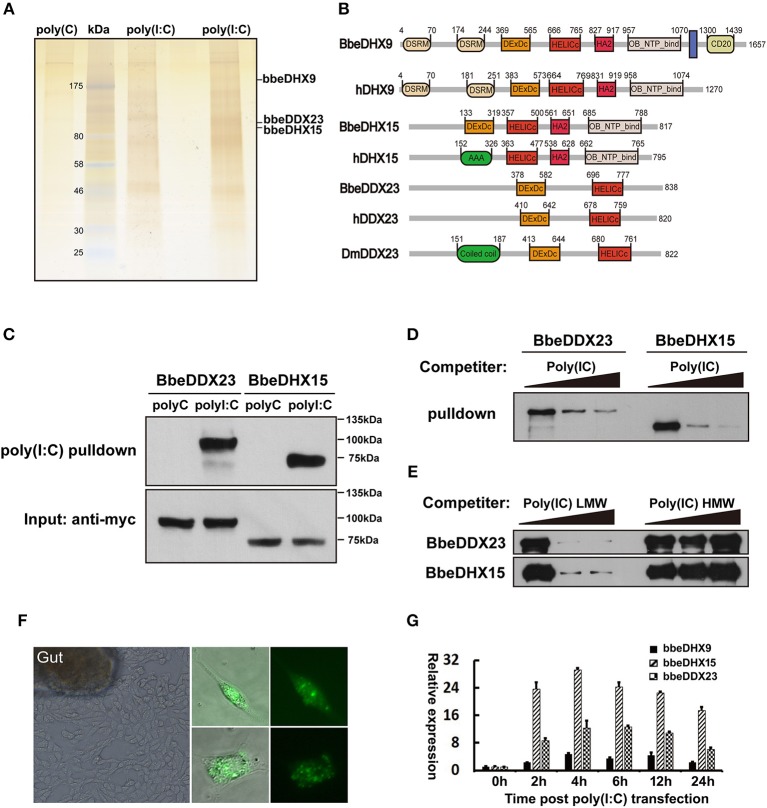
Figure 1.
Isolation of poly(I:C) binding proteins from amphioxus intestinal cells. (A) Silver stained poly(I:C)-associated proteins from amphioxus intestinal cells purified with the poly(I:C) agarose or control poly(C) agarose. Proteins identified by LC-mass spectrometry. Two poly(I:C) lines indicated samples from two separated experiments. (B) The comparison of protein architectures between Drosophila, amphioxus and human DDX23, DHX9, and DHX15. All these helicases have highly conserved DEXDc (DEAD-like helicases superfamily) and HELICc (helicase superfamily c-terminal) domains. (C) Pull-down assays were performed by incubating purified myc-bbeDHX15 or myc-bbeDDX23 with the poly(I:C) or poly(C) agarose. Bound proteins were analyzed by immunoblotting with anti-myc. (D) The mixture of myc-bbeDDX23 or myc-bbeDHX15 and poly(I:C) agaroses were incubated with or without free poly(I:C) at 0, 150, or 500 μg/ml. Bound proteins were analyzed by immunoblotting with anti-myc. (E) Pull-down competition assays using purified myc-bbeDDX23 or myc-bbeDHX15 in the presence of increasing amounts (0, 1,000, 3,000 μg/ml) of uncoupled low molecular weight (LMW) poly(I:C) or high molecular weight (HMW) poly(I:C). (F) Morphology of primary amphioxus intestinal cells in both resting and upon FITC-labeled poly(I:C) transfection. (G) Expression profiles of bbeDHX9, bbeDHX15, and bbeDDX23 were determined by RT-PCR using poly(I:C) transfected amphioxus intestinal cells. Data are shown as the means ± standard deviations of three samples per treatment. Values were considered to be significant when p < 0.05. The results were confirmed by at least three separate experiments.