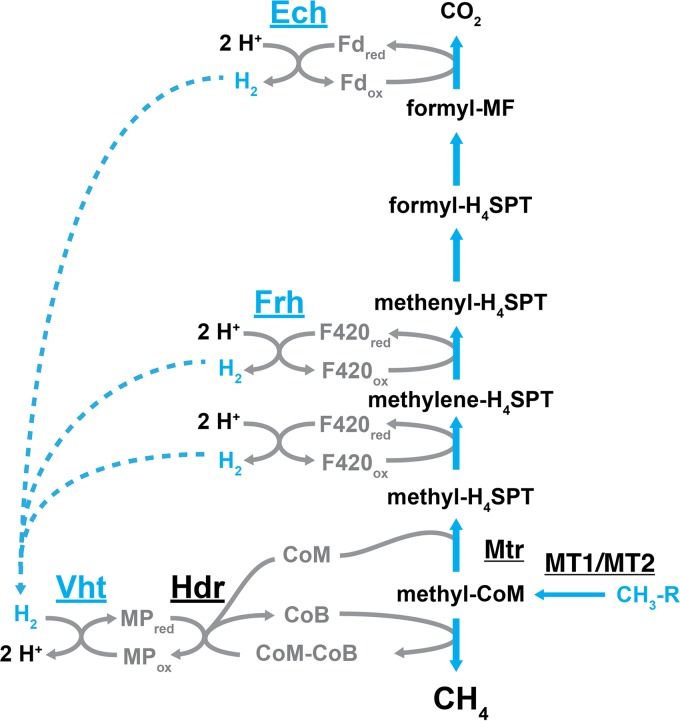
FIG 4.
The methylotrophic pathway of methanogenesis for M. barkeri. In this pathway, methyl compounds undergo disproportionation, wherein the oxidation of one methyl group to CO2 provides the reducing equivalents for the reduction of three additional methyl groups to CH4. In the oxidation portion of the pathway, methyl groups are first transferred to coenzyme M by the sequential action of methyltransferases MT1 and MT2, and then transferred to tetrahydrosarcinapterin by the methyl-H4SPT:CoM methyltransferase. The methyl group is oxidized in two successive steps, first to methylene-H4SPT and then to methenyl-H4SPT, which produces two reducing equivalents in the form of reduced coenzyme F420. The third reducing equivalent comes from the oxidation of formyl-methanofuran, which produces CO2 and reduced ferredoxin. In the reduction portion of the pathway, methyl-CoM is reduced by coenzyme B, which forms CH4 and a disulfide of CoM and CoB. Regeneration of CoM and CoB requires reduction of the disulfide by the heterodisulfide reductase enzyme with electrons from reduced methanophenazine. The transfer of electrons from the oxidative to reductive portions of the pathway occurs via a hydrogen cycling mechanism in M. barkeri. In this mechanism, F420red and Fdred are oxidized by Frh and Ech, respectively, which forms H2 on the inside of the cell. The H2 then diffuses across the membrane to the Vht active site on the outside of the cell, where it is oxidized and electrons are passed to MP, thereby completing the cycle. The methyl compound R groups include –OH (methanol), –NH2 (methylamine), and –SH (methanethiol). Other examples of methyl compounds include dimethylamine, trimethylamine, and dimethyl sulfide.