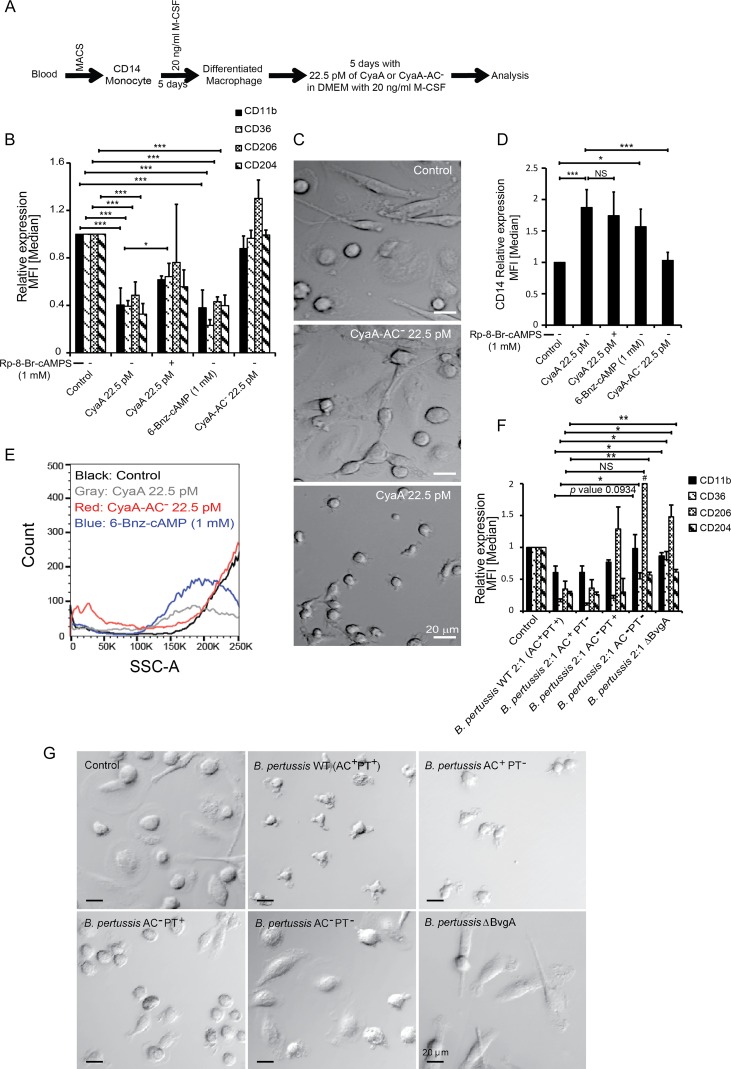
FIG 5.
CyaA-elicited cAMP signaling triggers dedifferentiation of fully differentiated macrophages. CD14+ monocytes were cultured for 5 days in DMEM–20 ng/ml M-CSF to induce differentiation into macrophages before 22.5 pM CyaA or CyaA-AC− was added, and the culture with 20 ng/ml M-CSF was continued for another 5 days in the presence of CyaA toxin. (A) Schematic depiction of the design of the dedifferentiation experiment. (B) Flow cytometry analysis of cell surface expression of macrophage markers on macrophages exposed for 5 days to CyaA or the indicated compounds. The cell-permeative and PKA-specific activator 6-Bnz-cAMP (1 mM) effectively elicited macrophage dedifferentiation. PKA inhibitor Rp-8-Br-cAMPS (1 mM) partially blocked CyaA toxin-triggered macrophage dedifferentiation. (C) Phase-contrast microscopy images of macrophages that were first differentiated for 5 days with 20 ng/ml M-CSF and then exposed for another 5 days to 22.5 pM CyaA toxin or CyaA-AC− toxoid in the continued presence of 20 ng/ml M-CSF. The shown images are representative of several dozens of images taken in 3 independent experiments (biological replicates). Bar, 20 μm. (D) CyaA-elicited cAMP signaling upregulated CD14 expression in M-CSF-matured macrophages. CyaA-AC− toxoid or mock-treated control cells maintained the CD14low phenotype of differentiated macrophages over 5 days of culture. Upon exposure of M-CSF-differentiated macrophages to the PKA-specific activator 6-Bnz-cAMP (1 mM), the CD14 expression level was upregulated. Exposure to the PKA inhibitor Rp-8-Br-cAMPS (1 mM) did not fully block CyaA toxin-triggered upregulation of CD14. *, P < 0.05; **, P < 0.005; ***, P < 0.0005. (E) M-CSF-differentiated macrophages lost intracellular complexity upon treatment with 22.5 pM CyaA or 1 mM PKA-specific activator 6-Bnz-cAMP for 5 days, as observed by low side scatter. Control and CyaA-AC−-exposed cells retained complex cytoplasm rich in cellular organelles. (F) M-CSF-differentiated macrophages were infected by wild-type and mutant strains of B. pertussis CIP 81.32 Tohama I at MOI of 2:1 for the first 12 h of culture. Bacteria were killed by the use of 50 μg/ml polymyxin B plus 50 μg/ml kanamycin over additional 12 h of incubation. 5 days later, after repeated medium replacement, the expression levels of macrophage markers was analyzed by flow cytometry. n = 3 biological replicates; *, P < 0.05; **, P < 0.005; #, above range. (G) Phase-contrast microscopy analysis of cell shape and appearance of mature macrophage cells after 5 days of dedifferentiation resulting following infection with B. pertussis for 12 h at MOI 2:1. Macrophages were first allowed to mature for 5 days with 20 ng/ml M-CSF in DMEM. Next, the macrophage cells were infected with the wild-type strain or the indicated mutant strains of B. pertussis CIP 81.32 Tohama I at MOI of 2:1 for 12 h. The bacteria were then killed with 50 μg/ml polymyxin B plus 50 μg/ml kanamycin over 12 h. The cells were further cultured for 5 days in DMEM–20 ng/ml M-CSF, with half of the media being replenished with fresh medium. After 5 days postinfection, cells were visualized by phase-contrast microscopy, where the macrophage cells exhibited altered morphology, drastic cell shrinkage, and reduced adherence to the surface of the plastic. The shown images are representative of several dozens of images taken on 3 biological replicates. Bar, 20 μm.