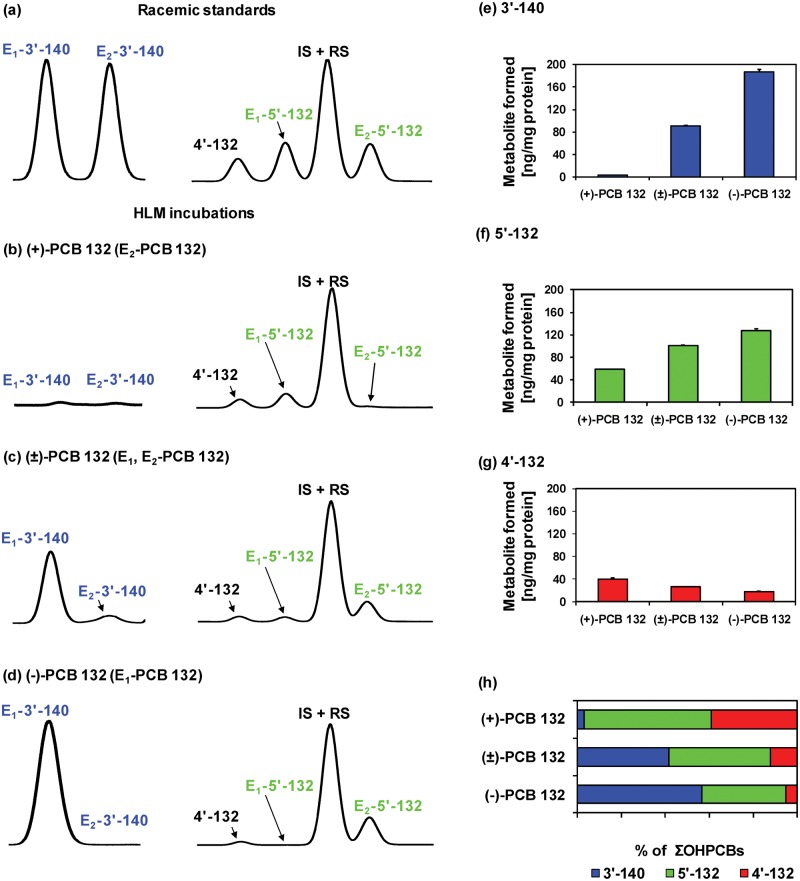
Figure 8.
Comparison of representative gas chromatograms of (a) racemic OH-PCB metabolite standards with OH-PCB metabolites formed in incubations of (b) (+)-PCB 132 (2,2′,3,3′,4,6′-hexachlorobiphenyl), (c) (±)-PCB 132, or (d) (−)-PCB 132 with pooled human liver microsomes (pHLMs) reveals that E1-5′-132 (2,2′,3,3′,4,6′-hexachlorobiphenyl-5′-ol) is formed from (+)-PCB 132 and E1-3′-140 (2,2′,3,4,4′,6′-hexachlorobiphenyl-3-ol), and E2-5′-132 are formed from (−)-PCB. Moreover, (e) 3′-140 and (f) 5′-132, but not (g) 2,2′,3,3′,4,6′-hexachlorobiphenyl-4′-ol (4′-132) are formed more rapidly from (−)-PCB 132 than (+)-PCB 132, resulting (h) in distinct OH-PCB metabolite profiles formed from (+)-, (±)-, and (−)-PCB 132 in incubations with pHLMs. Incubations were carried out with 50 µM (+)-PCB 132, racemic PCB 132, or (−)-PCB 132, 0.1 mg/ml microsomal protein and 1 mM NADPH for 30 min at 37°C (Uwimana et al., 2016). Metabolites were analyzed as the corresponding methylated derivatives after derivatization with diazomethane. Atropselective analyses of 3′-140 were performed with a ChiralDex G-TA (GTA) column at 150°C, and atropselective analyses of PCB 132 and 5′-132 were carried out with a CP-Chirasil-Dex CB (CD) column at 160°C. 4′-132 was not resolved on any of the columns used in this study (Kania-Korwel et al., 2011). Data are presented as mean ± standard deviation, n = 3.