Abstract
Objective(s):
Toxoplasma gondii is an obligate intracellular protozoan parasite that causes toxoplasmosis in humans and animals. Micronemes (MICs) are effective candidates for DNA vaccine.
Materials and Methods:
In this study, we evaluated the immune response of BALB/c mice against MIC3 gene of Toxoplasma gondii and interleukin 12 (IL-12) as DNA vaccine. The MIC3 gene was cloned into the PTZ57R/T vector before sub-cloning in pcDNA3. Recombinant pc-MIC3 was transformed into Escherichia coli (TOP10 strain). The pc-MIC3 plasmid was then transfected into Chinese Hamster Ovary (CHO) cells, and the expression of the MIC3 gene was evaluated by SDS-PAGE and Western blotting. Sixty female BALB/c mice were divided into 6 groups. Each group received 3 intramuscular immunizations on days 0, 21st and 42nd using one of the following stimulants: phosphate-buffered saline, pcDNA3, pCAGGS-IL12, pc-MIC3 (100 µg), pc-MIC3 (50 µg), or combined pCAGGS-IL12 (50 µg) and pc-MIC3 (50 µg). The enzyme-linked immunosorbent assays was applied to evaluate interferon gamma (IFN-γ) and IL-4 cytokines excretion of lymphocytes stimulated with tachyzoites lysate antigen, as well as the total levels of immunoglobulin G (IgG), IgG2a and IgG1 in immunized mice sera.
Results:
Our results showed that mice challenged with pc-MIC3 (100 µg) had the highest longevity and quantity of immunoglobulin. Moreover, the highest expression level of IFN-γ was found in mice injected with combined pcMIC3 and pCAGGS-IL12 (P<0.05).
Conclusion:
The MIC3 gene can be an efficient DNA vaccine candidate against toxoplasmosis. While, the single-gene vaccine can confer partial protection to mice against toxoplasmosis, the multigene vaccine can significantly enhance immune responses.
Key Words: BALB/c mice, DNA vaccine, Immunization, pCAGGS-IL12, pc-MIC3, Toxoplasma gondii
Introduction
Toxoplasmosis is one of the most common parasitic infections caused by protozoa in the world. The causative agent of this disease is an obligate intracellular protozoan called Toxoplasma gondii that belongs to the phylum of Apicomplexa (1). This parasite infects not only humans but also a large number of mammals, as well as different types of birds. It can replicate in host cell in three forms of tachyzoites, bradyzoites (tissue cyst) and sporozoites (oocysts) (2). The parasite can cause severe neurological and eye complications in immunocompromised patients, whereas in infants born to infected mothers may result in hydrocephalus, microcephaly, calcification, or chorioretinitis (3-5). Given its widespread distribution, easy transmission and irreparable complications, toxoplasmosis needs to be prevented by anti-parasitic drugs, or vaccination (6). Today, immunization by plasmids containing DNA sequences encoding favorable antigens has opened a new horizon in vaccine production (7, 8). Some antigens of T. gondii including rhoptry proteins (ROPs), surface antigen glycoproteins (SAGs), excretory-secretory dense granule proteins (GRAs) and microneme proteins (MICs) are relatively effective candidates for DNA vaccine (9-11). The production of MIC proteins in microneme organelles such as prerequisite proteins for promotion of adhesion and invasion by T. gondii makes them suitable antigens for DNA vaccine production. The MIC3 protein plays an important role in identification of T. gondii and attachment to the host cells (12). Different studies showed that MIC3 is very immunogenic besides being encoded by a single-gene lacking introns (13). It is known as the main T. gondii antigen, which stimulates immunity response, hence attracting more immunological investigations (14-15). In the present study, MIC3 gene was cloned and expressed in Chinese Hamster Ovary (CHO) cells. Also, immunization with a DNA plasmid encoding T. gondii MIC3 was assessed in BALB/c mice.
Materials and Methods
Ethics statement
This project was approved by the Medical Ethics Committee of Tarbiat Modares University, Tehran, Iran, adopting the Declaration of Helsinki (1975) and Guidelines of the Society for Neuroscience concerning Animal Care and Use (1998).
Animals and parasite
Female BALB/c mice aged 8 weeks were bought from Razi Vaccine and Serum Research Institute (RVSRI). In this study, we used the tachyzoites (RH strain) of T. gondii that was stored at -80 °C in the Parasitology Department of Tarbiat Modares University, Tehran, Iran.
Gene amplification of MIC3
DNA from parasite samples was extracted by phenol-chloroform and quantified by spectrophotometer using A260/280 ratio (16). The sequence of MIC3 gene was obtained from the gene bank (www.ncbi.nlm.nih.gov) with the accession number AJ132530. Both reverse and forward primers were designed using the Gene Runner software (Table 1).
Table 1.
Designing reverse and forward primers of microneme (MIC) gene with HindIII and EcoRV sequences that were added to reverse and forward primers, respectively
| Primer | The restriction enzymes site that added to primers | Nucleotide No | Sequence of Primers |
|---|---|---|---|
| Forward | HindIII | 31 nucleotides | 5΄- CACA ↓ A GCTTATGGCGCTCACCTTCATGGGGG - 3΄ |
| Reverse | EcoRV | 32 nucleotide | 5΄- ACAGAT ↓ ATCTCACGTCACGGTGTGGGCATGGT - 3΄ |
The PCR reaction was performed in a volume of 25 μl. MIC3 gene was amplified by PCR during 35 cycles according to the following program: pre-denaturation at 94 °C for 5 minutes, followed by 35 cycles at 94 °C for 1 min, 56 °C for 30 sec and 72 °C for 50 sec, and final extension for 7 minutes at 72 °C. The PCR product was analyzed by electrophoresis on 1% agarose gel. DNA was purified from the agarose gel with the aid of Agarose Gel DNA Extraction Kit (Bioneer, Germany) as per Sambrook et al. 2001 (17).
Cloning of MIC3 gene and construction of recombinant plasmids
MIC3 gene was cloned in pTZ57R/T vector by InsTAcloneTM PCR cloning Kit (Fermentas, Lithuania). Bacteria (Escherichia coli TG1 strain) were prepared from culture containing calcium chloride before being transformed by the thermal shock in the presence of pT-MIC3. Bacteria were then cultured in LB (Luria Bertani) broth containing ampicillin antibiotic, X-gal, and isopropyl β-D-1-thiogalactopyranoside (IPTG). Plates were incubated at 37 °C (16-18 hr. overnight). The plates were then placed at 4 °C for either blue- (lacking pT-MIC3) or white-colony formation (containing pT-MIC3). The white colonies were collected from the cultures and the gene cloning was confirmed via PCR and sequencing methods (17).
Sub-cloning of MIC3 gene
Two plasmids of pT-MIC3 and pcDNA3 were digested with HindIII and EcoRV enzymes, and the products were analyzed by electrophoresis on 1% agarose gel. The MIC3 gene and pcDNA3 plasmid were then purified from agarose gel using a DNA extraction kit. The MIC3 gene was ligated to pcDNA3 plasmid with aid of T4DNA ligase enzyme. The resulting pcMIC3 was transformed into the E. coli (TG1 strain) using a thermal shock. The bacteria were then cultured in a tube containing LB liquid and ampicillin at 37 °C (16-18 hrs overnight). To confirm gene cloning, enzymatic digestion process was performed using EcoRV and Hind III enzymes (17).
Expression of pc-MIC3 in CHO cells
For this experiment, 1-3×106 CHO cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing fetal calf serum (FCS) 10% and 100 unit/ml of penicillin in addition to 100 mg/ml of streptomycin and incubated overnight in 5% CO2 at 37 °C. The Fugen 6 Transfection Reagent Kit (Roche, USA) was used to transfect pc-MIC3 to the CHO cells. The product of MIC3 gene was extracted from CHO cells by sonication and freeze-thaw technique. The density of MIC3 product was measured by Bradford method (18).
SDS-PAGE and Western blotting
The molecular weight of the protein was evaluated through SDS-PAGE (19, 20). For Western blotting, the constructed protein bands were transferred to nitrocellulose paper and recognized by sera positive against toxoplasmosis, goat Anti-Human immunoglobulin G (IgG) antibody, horseradish peroxidase (HRP) conjugate (Sigma-Aldrich) and 3,3′-Diaminobenzidine (DAB) (Sigma-Aldrich) as the substrate (20).
BALB/c mice immunization and challenge
Immunization was assessed in sixty BALB/c mice, which had been divided into 6 groups (10 mice per each group). Three groups were allocated to control treatments, each received one intramuscular injection of phosphate-buffered saline (PBS; 100 μl), pcDNA3 (100 μg) or pCAGGS-IL12 (100 μg) in the quadriceps femoris muscle. Each of the three main experimental groups was administered with one injection of pc-MIC3 plasmid (100 μg), combined pcMIC3 plasmid (50 μg) and pCAGGS-IL12 plasmid (50 μg) or combined pc-MIC3 plasmid (50 μg) and pcDNA3 plasmid (50 μg). All mice received the same volume for injection that was equal to 100 μl. The immunization was undertaken at 3-week intervals on days 0, 21, and 42. Three weeks after the last immunization on day 63, mice were challenged with 1×104 tachyzoites (RH strain) recovered from peritoneal fluid. The experimental mice were then monitored daily to determine the fatality rates of different groups based on their protection against the disease (21).
Humoral immunity
From each group, 5 mice were randomly selected and bled from eyes on days 21, 42, and 63. The mice sera were collected and examined against toxoplasmosis by ELISA technique. The concentration of parasite antigen tachyzoites lysate antigen (TLA) was 20 µg/ml, and sera dilution 1:10 were used for ELISA. Also, subtypes of IgG antibody were measured by Monoclonal Antibody Isotyping Reagents Kit, and Capture ELISA method (22, 23).
Cellular immunity
Seven weeks after the third immunization, half of mice in each group were sacrificed and their spleens were scooped to prepare lymphocytes. The spleen lymphocytes were cultured in RPMI with 10% fetal bovine serum (FBS). The cells viability was tested by trypan blue. The lymphocytes (2×106/ml) were incubated with TLA (20 µg/ml) for 72 hrs in 5% CO2 at 37 °C. The supernatant of cell cultures were then collected for cytokine assay. For evaluation of interleukin 4 (IL-4) and interferon gamma (IFN-γ) of cytokines, the supernatant was investigated by ELISA kit (Ucytech, Netherland). The standard curve was created for each kit, and the measurements of both IL-4 and IFN-γ cytokines were carried out according to the manufacturer’s instruction (23).
Statistical analysis
Statistical tests of Kruskal-Wallis, Kaplan Meiere, and Mann-whitne were applied to analyze the data using SPSS version 19. The results were reported as mean±standard deviation for each group. In all statistical analyses, the difference was considered significant at P<0.05 (16, 17).
Results
PCR amplification of MIC3 and sequence determination
Figure 1 shows that PCR amplified DNA has about 1052 bp, equals to the size of toxoplasma MIC3 gene. Also, no gene has been amplified except MIC3. Therefore, the primers were found to be designed specifically for MIC3 gene amplification.
Figure 1.
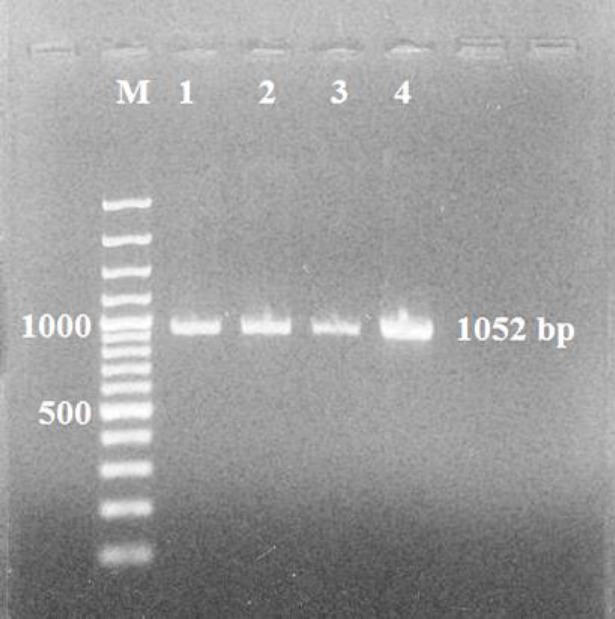
Electrophoresis of microneme 3 (MIC3) PCR product (1052 bp) equals to the size of toxoplasma MIC3 gene in comparison with 100 bp DNA ladder on agarose gel
The sequence analysis of MIC3 gene of T. gondii in pTZ57R/T plasmid showed 100%, 99% and 98% similarity with Genbank-registered MIC3 gene of T. gondii (RH Strain) with the accession numbers of JF330835.1, DQ676961.1 and AJ132530.1, respectively.
Construction of pcDNA-MIC3 plasmid
Figure 2 shows enzymatic digestion of pTMIC3 and pcDNA3 by EcoRV and HindIII enzymes. After separation of MIC3 gene from pT-MIC3, the MIC3 gene was sub-cloned in pcDNA3 plasmid. On agarose gel, the bands of pc-MIC3 plasmid were above those of pcDNA3 plasmid. This indicates that pcMIC3 plasmid is heavier than pcDNA3 plasmid; hence, the cloning of MIC3 portion of pcDNA3 plasmid was ensured. The formation of two bands of 5.4 kbp (equal to pcDNA3 weight without MIC3) and 1052 bp (equal to MIC3 weight) showed that this plasmid was cut into two portions by EcoRV and HindIII enzymes.
Figure 2.
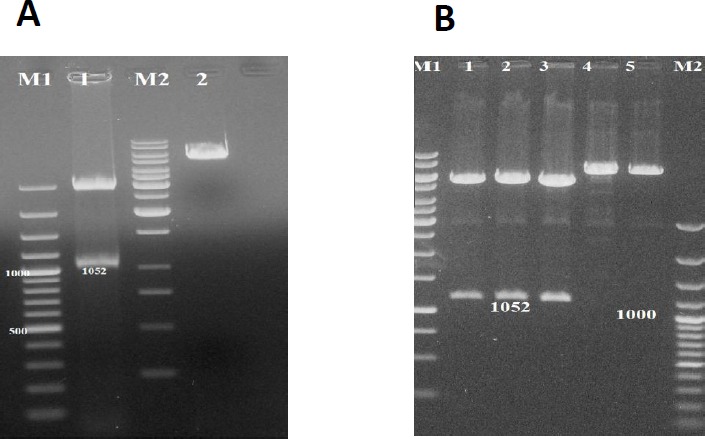
A: Electrophoresis of M1, 100 bp DNA ladder; Line 1, pT-MIC3 with double digestion with EcoRV and HindIII enzymes; M2, 250 bp DNA ladder; Line 2, pT-MIC3 with one digestion on agarose gel. B: Double digestion of pc-MIC3 (Lines 1-3) with EcoRV and HindIII enzymes and one digestion of pc-MIC3 (Lines 4-5) with EcoRV or HindIII enzymes, M1, 100 bp DNA ladder; M2, 250 bp DNA ladder
Gene expression in CHO cells
SDS-PAGE (Figure 3) showed that the loaded proteins were separated based on their molecular weights within the range of 14 to 116 kDa. The formation of protein band with molecular weight of about 40 kDa on nitrocellulose paper showed that MIC3 protein may be identified by Western blotting (Figure 4) with human anti-Toxoplasma IgG positive sera (24).
Figure 3.
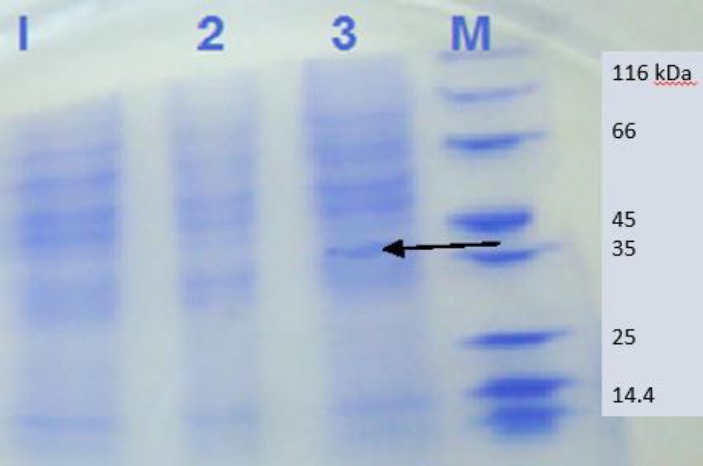
SDS-PAGE of transfected Chinese Hamster Ovary (CHO) cells with pc-MIC3 (line 3), pc-DNA (line 2) and empty CHO cells (line 1) in comparison with protein ladder (M)
Figure 4.
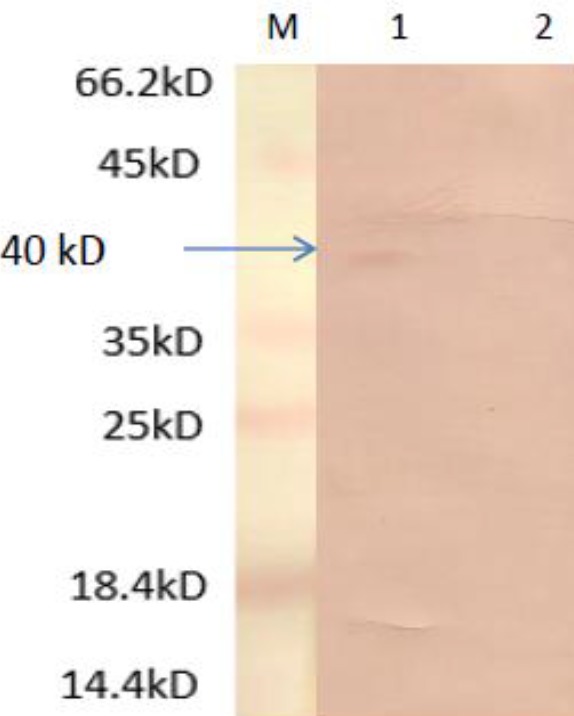
The result of Western blotting of transfected Chinese Hamster Ovary (CHO) cells with pc-MIC3 (line 1), and empty CHO cells (line 2) in comparison with protein ladder (M)
BALB/c mice immunization and challenge
The results showed that the three control groups treated with pcDNA3, PBS or pCAGGS-IL12 experienced mortality from day 4 post-challenge until day 6. All mice succumbed to death by day 6. However, for pcDNA3 and pcIL12 groups the survival rates were 40% at the end of 4th day and zero at the end of 5th day. In vaccinated experimental groups, the mortality of mice began from day 6 post-challenge for those immunized with pcMIC3/pCAGGS-IL12 and pcMIC3/pcDNA3, and from day 7 for pcMIC3 group. The pcMIC3/pcDNA3-challenged mice remained alive until the end of day 8, though the pcMIC3+pCAGGS-IL12- challenged mice survived until day 10. In term of survival rates, there were significant differences between those immunized with pcMIC3 (100 µg) and pcMIC3+pcIL12 and control groups (P<0.05) (Figure 5).
Figure 5.
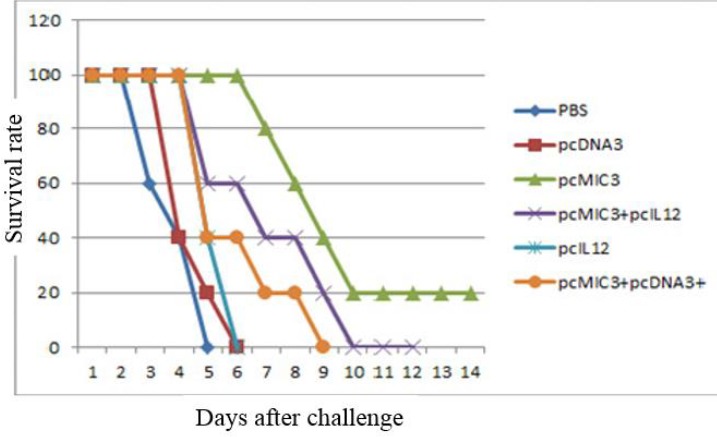
The survival rate of vaccinated BALB/c mice after challenge with 104 live Toxoplasma gondii (RH strain) tachyzoites. There is significant differences for pcMIC3 (100 µg) and pcMIC3+pcIL12 with control groups by Kaplan–Meier test (P<0.05)
Humoral immunity
The measurement of IgG
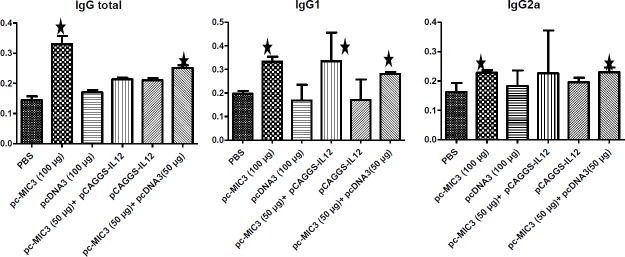
Among the tested group, the PBS and pcMIC3 groups expressed very low and very high levels of IgG both in the first and second blood sampling, respectively. Significant differences in IgG levels (P ≤0.05) were also observed between the first and second blood sampling of each group (Figure 6).
Figure 6.
Mean±SD the optical density (OD) of total immunoglobulin G (IgG) and IgG1, IgG2a in mice sera of immunized and control groups
* There is significant differences with control groups (P<0.05)
The measurement of IgG subtypes (IgG 1 , IgG 2a )
The lowest and highest level of IgG1 was recorded in pcMIC3/pCAGGS-IL12, and pcDNA3 groups, respectively. However, the titers of IgG2a were higher in pcMIC3/pCAGGS-IL12 group and lower in PBS group (Figure 6).
Cellular immunity
The measurement of IFN-γ and IL-4 cytokines
After 72 hrs of lymphocyte culture, the highest and lowest amounts of IL-4 cytokine were found in PBS and pcMIC3/pCAGGS-IL12 groups, respectively. Whereas, the highest and lowest levels of IFN-γ cytokine were recorded in pcMIC3/pCAGGS-IL12 and PBS groups, respectively (Figure 7).
Figure 7.
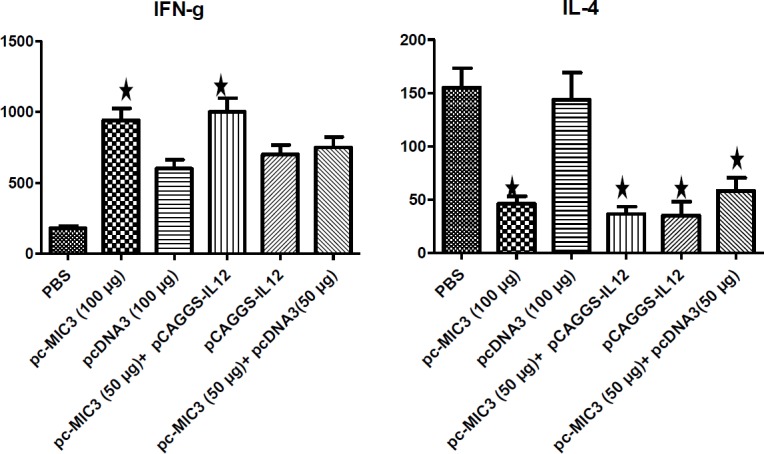
Mean ±SD of interleukin 4 (IL-4) and interferon gamma (IFN-γ) amount after culture of lymphocyte in 72 hrs in immunized mice of experimental and controlled group.* There is significant differences with control groups (P<0.05)
Discussion
DNA vaccination is a powerful method for induction of cellular and humoral immune responses. Upon injection of DNA plasmid into the host, the host cells express the encoded protein (23). In general, it should be mentioned that DNA vaccination induces type 1 T helper (Th1) immune responses more than those of Th2 (24, 25). The vaccination efficiency depends more on the way of producing immunity. DNA vaccines are highly immunogenic and induce different immunity responses compared to normal ones (26). In these vaccines, DNA itself acts as an adjuvant. The existence of special sequence of DNA, enriched with non-methylated CpG motif in vector or added to vaccine formula, will intensify the response of Th1 pattern, and induce the actions of IL-12, IL-6 and IFN-γ (27). Previous studies about prophylaxis of toxoplasmosis disease showed that vaccination with excretory-secretory antigens of T. gondii like ROPs, GRAs, and MICs can create multifaceted immune responses against the parasite (10, 11). In this study, the immunity production of a plasmid encoding T. gondii MIC3 in BALB/c mice was investigated. The plasmid encoding MIC3 with or without adjuvant of IL-12 was injected in BALB/c mice. This study showed that IL-12 adjuvant improved survival rates of mice.
The pc-MIC3 plasmid showed the ability to prolong the survival of vaccinated mice either with or without adjuvant, although it worked better when injected at 100 µg rather than at 50 µg, even if combined with IL-12 adjuvant. The level of immune response stimulation by an adjuvant depends on its composition and the way of its formulation with MIC3. Xue and his colleagues examined a multi antigen DNA vaccine expressing ROP2, and SAG1 against toxoplasmosis along with subunit of cholera toxin and IL-12 as the genetic adjuvant in BALB/c mice. They showed that multi antigen vaccine combined with IL-12 adjuvant elicited higher humoral and cellular (Th1) responses in mice compared to the stand-alone multi antigen vaccine (8). Xue evaluated a DNA vaccine containing SAG1, ROP2, and GRA2 of Toxoplasma in mice and reported higher Th1 responses by a plasmid containing 3 genes compared to that containing 2 genes. Based on the properties of genetic adjuvants (IL-12), we expected that the level of antibody in groups challenged with pc-MIC3 plasmid plus adjuvant will be higher than those received pc-MIC3 plasmid alone. However, we observed no increase of antibody in mice injected with pc-MIC3 plasmid along with IL-12 both at first and second phases of blood sampling. Desolmi stated that immunization of mice with concurrent administration of IL-12 and GRA4 plasmids resulted in an antagonistic effect and therefore decreased mice survival (28). Furthermore, the concurrent use of the plasmid and IL-12 as an adjuvant caused increase of immunity responses and survival in mice (29). Assessing a multi antigen vaccine, Xue and his colleagues showed that the vaccine had reduced efficacy unless its main genes were combined in fused form and applied with adjuvant (29). The results of the present study revealed that the pc-MIC3 plasmid can solely activate the immunity system and induce production of appropriative antibody. Addition of genetic adjuvant to a fusion form may have even more effects. Investigations of IL4 and IFN-γ cytokines showed that the pc-MIC3 plasmid can stimulate cellular immunity. The high production of IFN-γ activates macrophages and hinders the growth of parasites by demolishing tryptophan and actuating inducible nitric oxide synthase (iNOS) mechanism. Therefore, lymphokines and macrophages play important roles in immunity response of Th1 (30, 31). The reduced amount of IL4 in this study provided evidence in this connection. In terms of production of high quantity of IFN-γ and low quantity of IL-4, our findings were in conformity with Xue’s study, who used multi antigens containing 2 or 3 genes of GRA2, SAG1, and ROP2 (29). The same was fund by Vercommen, using ROP2, GRA1, and GRA7 (14) and by Dautu et al. using plasmids containing bradyzoite antigen-1 (BAG1), apical membrane antigen 1 (AMA1), microneme protein-2 associated protein (M2AP) and MIC2 (27). The present study revealed that the first phase of pcMIC3’s vaccination elicited quick humoral responses and become more effective over time upon further vaccination. Xue and co-workers challenged two groups of mice with either of the two plasmids pSAG-ROP2-GRA2 and pSAG-ROP2 and reported that the former vaccine produced higher amount of IgG2a and therefore stronger Th1 response (29). Moreover, Desolme et al. stated that both pcGRA4 and pcGRA4/pGM-CSF plasmids elicited increased production of IgG in mice with no significant differences in their efficacy (28). Taking into account, it seems that application of MIC3 and pCAGGS-IL12 conjugate can result in increased immunity responses that are related to Th1. Studies showed that genetic adjuvants increase antibody production, so that cytokines containing plasmids modulate immune responses towards actuation and development of Th1 (IFN-γ), whereas using recombinant proteins of antigens as adjuvant actuates Th2 (IL-4) type. Yang et al. found that recombinant TgMIC3 has a cross protective effect against both T. gondii and Neospora caninum (32).
In recent studies, we showed the importance of MIC antigens in the progress and development of vaccines (33, 34). In a study, Ismael et al. showed that a DNA vaccine containing MIC3 significantly increased cellular immunity responses and secretion of IFN-γ and IL-2 cytokines in immunized mice. They also used a plasmid that encoded granulocyte-macrophage colony-stimulating factor (GM-CSF) as adjuvant (35-38). In the present study, we observed increase in cellular immune responses as a result of designed DNA vaccine, especially when we used IL-12 as a genetic adjuvant.
Conclusion
The results of the present study showed that immunity responses, specifically the cell immunity were constructed in mice after immunization with pc-MIC3 (in combination with IL-12 as a genetic adjuvant). However, this could not provide complete protection to mice against the virulent strain of T. gondii.
Acknowledgment
The results described in this paper were part of student thesis. The authors would like to acknowledge the financial support of Tarbiat Modares University, Tehran, Iran. They also wish to sincerely appreciate the assistance of all members of Parasitology Department. The authors are thankful to Dr Masanori Matsui from the University of Tokyo, Japan.
Conflicts of interest
The authors declare that there are no conflicts of interest.
References
- 1.Rosado-Vallado M, Mut-martiin M, Garcia-Miss M, Dumonteil E. Aluminum phosphate potentiates the efficacy of DNA vaccines against Leishmania mexicana. Vaccine. 2005;23:5372–5379. doi: 10.1016/j.vaccine.2005.05.037. [DOI] [PubMed] [Google Scholar]
- 2.Saeb A. Parasitic diseases in Iran. Vol. 6. Tehran: Hayyan Publishing; 1996. p. 123. [Google Scholar]
- 3.Dziadek B, Gatkowska J, Brzostek A, Dziadek J, Dzitko K, Dlugonska H. Toxoplasma gondii: The immunogenic and protective efficacy of recombinant ROP2 and ROP4 rhoptry proteins in murine experimental toxoplasmosis. Exp Parasitol. 2009;123:81–89. doi: 10.1016/j.exppara.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Wang Qu D, Cai S, Du W A. Protective effect of a DNA vaccine delivered in attenuated Salmonella typhimurium against Toxoplasma gondii infection in mice. Vaccine. 2008;26:4541–4548. doi: 10.1016/j.vaccine.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 5.Tan TG, Mui E, Cong H, Witola WH, Montpetit A, Muench SP, et al. Identification of T gondii epitopes adjuvants and host genetic factors that influence protection of mice and humans. Vaccine. 2010;28:3977–3989. doi: 10.1016/j.vaccine.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, He S, Jiang H, Yang T, Cong H, Zhou H, et al. Evaluation of the immune response induced by multiantigenic DNA vaccine encoding SAG1 and ROP2 of Toxoplasma gondii and the adjuvant properties of murine interleukin-12 plasmid in BALB/c mice. Parasitol Res. 2007;101:331–338. doi: 10.1007/s00436-007-0465-3. [DOI] [PubMed] [Google Scholar]
- 7.Yang X, Huang S, Chen J, Song N, Wang L, Zhang Z, et al. Evaluation of the adjuvant properties of Astragalus membranaceus and Scutellaria baicalensis GEORGI in the immune protection induced by UV-attenuated Toxoplasma gondii in mouse models. Vaccine. 2010;28:737–743. doi: 10.1016/j.vaccine.2009.10.065. [DOI] [PubMed] [Google Scholar]
- 8.Xue M, He S, Cui Y, Yao Y, Wang H. Evaluation of the immune response elicited by multi antigenic DNA vaccine expressing SAG1, ROP2 and GRA2 against Toxoplasma gondii. Parasitol Int. 2008;57:424–429. doi: 10.1016/j.parint.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Meng M, Zhou A, Lu G, Wang L, Zhao G, Han Y, et al. DNA prime and peptide boost immunization protocol encoding the Toxoplasma gondii GRA4 induces strong protective immunity in BALB/c mice. BMC Infect Dis. 2013;13:494. doi: 10.1186/1471-2334-13-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang NZ, Chen J, Wang M, Petersen E, Zhu XQ. Vaccines against Toxoplasma gondii: new developments and perspectives. Expert Rev Vaccines. 2013;12:1287–1299. doi: 10.1586/14760584.2013.844652. [DOI] [PubMed] [Google Scholar]
- 11.Garcia JL, Innes EA, Katzer F. Current progress toward vaccines against Toxoplasma gondii. Vaccine Dev Therap. 2014;4:23–28. [Google Scholar]
- 12.Cerede O, Dubremetz JF, Soete M, Deslee D, Vial H, Bout D, et al. Synergistic role of micrinemal proteins in Toxoplasma gondii virulence. J Exp Med. 2005;201:453–63. doi: 10.1084/jem.20041672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garcia-Reguet N, Lebrun M, Fourmaux MN Mercereau-Puijalon O, Mann T, Beckers CJM, et al. The microneme protein MIC3 of Toxoplasma gondii is a secretory adhesin that binds to both the surface of the host cells and the surface of the parasite. Cell Microbiol. 2000;2:353–364. doi: 10.1046/j.1462-5822.2000.00064.x. [DOI] [PubMed] [Google Scholar]
- 14.Vercommen M, Scorza T, Huygen K, Braekeleer JD, Diet R, Jacobs D, et al. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, ROP2 induce partially protective immunity against lethal challenge in mice. Infect Immun. 2000;68:38–45. doi: 10.1128/iai.68.1.38-45.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harper JM, Hoff EF, Carruthers VB. Multimerization of the Toxoplasma gondii MIC2 integrin-Like A-domain is required for binding to heparin and human cells. Mol Biochem Parasit. 2004;134:201–212. doi: 10.1016/j.molbiopara.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Chai JY, Lin A, Shin EH, Oh MD, Han ET, Nan HW, et al. Laboratory passage and characterization of an isolate of Toxoplasma gondii from an ocular patient in Korea. Korean J parasitol. 2003;41:137–54. doi: 10.3347/kjp.2003.41.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning a laboratory manual. 3rd Ed. New York: Cold Spring Harbor Laboratory Press; 2001. pp. 1–32. [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Bioch. 1979;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Mostafaei AS. Guide gel electrophoresis protein. Second Edition. Yadavran nashr; 2001. [Google Scholar]
- 21.Sasaki S, Takeshita F, Xin K-Q, Ishii N, Okuda K. Adjuvant formulations and delivery systems for DNA vaccines. Methods. 2003;31:234–254. doi: 10.1016/s1046-2023(03)00140-3. [DOI] [PubMed] [Google Scholar]
- 22.Kravdr JR. ELISA theoretical and practical. The ELISA Guidebook. Totowa, New Jersey: Humana press; 2002. [Google Scholar]
- 23.Garcia JL, Gennari SM, Navarro IT, Machado RZ, Sinhorini IL, et al. Partial protection against tissue cysts formation in pigs vaccinated with crude rhoptry proteins of Toxoplasma gondii. Vet Parasitol. 2005;129:209–217. doi: 10.1016/j.vetpar.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Naserifar R, Ghaffarifar F, Dalimi A, Sharifi Z, Solhjoo K, Hosseinian Khosroshahi K. Evaluation of immunogenicity of cocktail DNA vaccine containing plasmids encoding complete GRA5, SAG1, and ROP2 antigens of Toxoplasma gondii in BALB/C mice. Iran J Parasitol. 2015;10:590–598. [PMC free article] [PubMed] [Google Scholar]
- 25.Tighe , H , Corr M, Roman M, Raz E. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol Today. 1998;19:89–97. doi: 10.1016/s0167-5699(97)01201-2. [DOI] [PubMed] [Google Scholar]
- 26.Letscher-Bru V, Pfaff AW, Abou-Bacar A, Filisetti D, Antoni E. Vaccination with Toxoplasma gondii SAG-1 Protein is protective against congenital Toxoplasmosis in BALB/C mice but not in CBA/J mice. Infec Immun. 2003;71:6615–6619. doi: 10.1128/IAI.71.11.6615-6619.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dautu G, Munyaka B, Carmen G, Zhang G, Omata Y, Xuenan X, et al. Toxoplasma gondii: DNA vaccination with genes encoding antigens MIC2, M2AP, AMA1 and BAG1 and evaluation of their immunogenic potential. Exp Parasitol. 2007;116:273–282. doi: 10.1016/j.exppara.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Desolme B, Mevelec MN, Gatel BD, Bout D. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii GRA4 gene. Vaccine. 2000;18:2512–2521. doi: 10.1016/s0264-410x(00)00035-9. [DOI] [PubMed] [Google Scholar]
- 29.Xue M, He S, Zhang J, Cui Y, Yao Y, Wang H. Comparison of cholera toxin A2/B and murine interleukin-12 as adjuvants of Toxoplasma multiantigenic SAG1-ROP2 DNA vaccine. Exp Parasitol. 2008;119:352–357. doi: 10.1016/j.exppara.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 30.Louis M, Weiss K. Toxoplasma gondii The Model Apicomplexan Perspectives and Methods. 1st ed. London: Elsevier Ltd; 2007. [Google Scholar]
- 31.Schwartzman JD. Toxoplasmosis. Curr Infect Dis Rep. 2001;3:85–89. doi: 10.1007/s11908-001-0063-y. [DOI] [PubMed] [Google Scholar]
- 32.Yang D, Liu J, Hao P, Wang J, Lei T, Shan D, et al. MIC3, a novel cross-protective antigen expressed in Toxoplasma gondii and Neospora caninum. Parasitol Res. 2015;114:3791–3799. doi: 10.1007/s00436-015-4609-6. [DOI] [PubMed] [Google Scholar]
- 33.Foroutan M, Zaki L, Ghaffarifar F. Recent progress in microneme-based vaccines development against Toxoplasma gondii. Clin Exp Vaccine Res. 2018;7:93–103. doi: 10.7774/cevr.2018.7.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghaffarifar F. Strategies of DNA vaccines against toxoplasmosis. Rev Med Microbiol. 2015;26:88–90. [Google Scholar]
- 35.Ismael AB, Sekkai D, Collin C, Bout D, Mevelec MN. The MIC3 gene of Toxoplasma gondii is a novel potent vaccine candidate against toxoplasmosis. Infect Immun. 2003;71:6222–6228. doi: 10.1128/IAI.71.11.6222-6228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismael AB, Hedhli D, Cerede O, Lebrun M, Dimier-Poisson I, Mevelec MN. Further analysis of protection induced by the MIC3 DNA vaccine against T gondii: CD4 and CD8 T cells are the major effectors of the MIC3 DNA vaccine-induced protection, both Lectin-like and EGFlike domains of MIC3 conferred protection. Vaccine. 2009;27:2959–2966. doi: 10.1016/j.vaccine.2009.02.107. [DOI] [PubMed] [Google Scholar]
- 37.Foroutan M, Ghaffarifar F, Dalimi A, Sharifi Z, Jorjani O. Rhoptry antigens as Toxoplasma gondii vaccine target. Clin Exp Vaccine Res. 2019;8:4–26. doi: 10.7774/cevr.2019.8.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ghaffarifar F. Plasmid DNA vaccines: where are we now? Drugs Today . 2018;54:315–333. doi: 10.1358/dot.2018.54.5.2807864. [DOI] [PubMed] [Google Scholar]