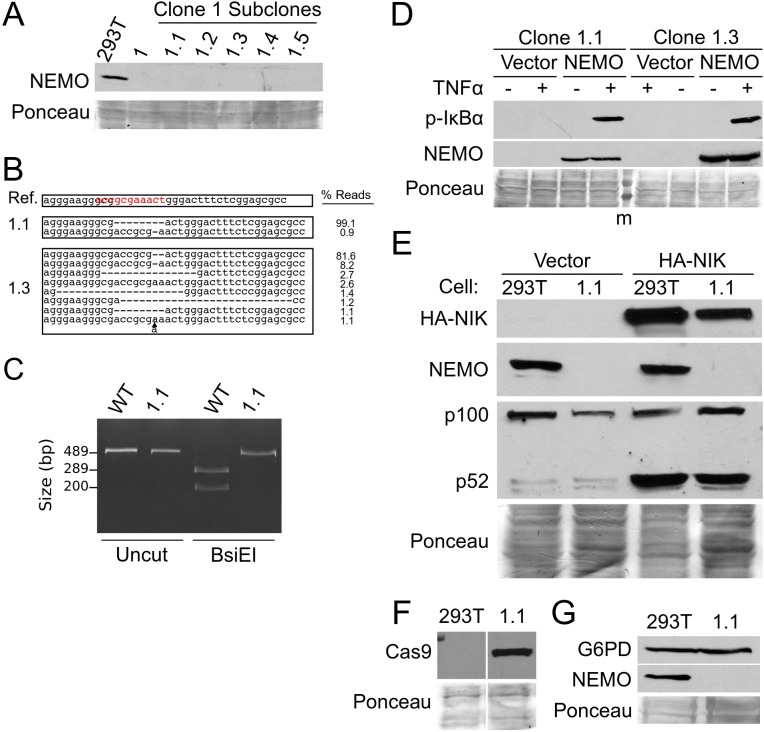
Fig 4. Isolation of cell subclones from clone 1 cells.
(A) Five subclones of clone 1 cells were isolated and analyzed by Western blotting for NEMO. Shown also are control 293T and clone 1 cell extracts. (B) CRISPR sequencing of the PCR-amplified exon 1B core promoter in clones 1.1 and 1.3 was used to characterize the genomic disruptions (as done for Fig 2C). (C) PCR-amplified exon 1B core promoter from WT 293T and clone 1.1 genomic DNA was analyzed as Uncut or BsiE1-digested DNA. DNA was electrophoresed on a 1.5% agarose gel and detected with ethidium bromide. (D) Clone 1.1 and 1.3 cells were transfected with pcDNA-FLAG or pcDNA-FLAG-NEMO. Two days later, cells were treated with TNFα (+, 20 ng/ml for 10 min) or were left untreated (-). Whole-cell extracts were analyzed for phospho-IκBα or NEMO expression. (E) Control 293T cells and clone 1.1 cells were transfected with pcDNA vector control or pcDNA-HA-NIK. Whole-cell extracts were subjected to Western blotting for HA-NIK, NEMO, and p100/p52. (F) Control 293T cells and clone 1.1 cell extracts were analyzed by Western blotting for FLAG-Cas9 (anti-FLAG). (G) Control 293T cells and clone 1.1 cell extracts were analyzed by Western blotting for G6PD and NEMO. Where indicated, Ponceau staining of the filters was done to ensure approximately equal loading of total protein.