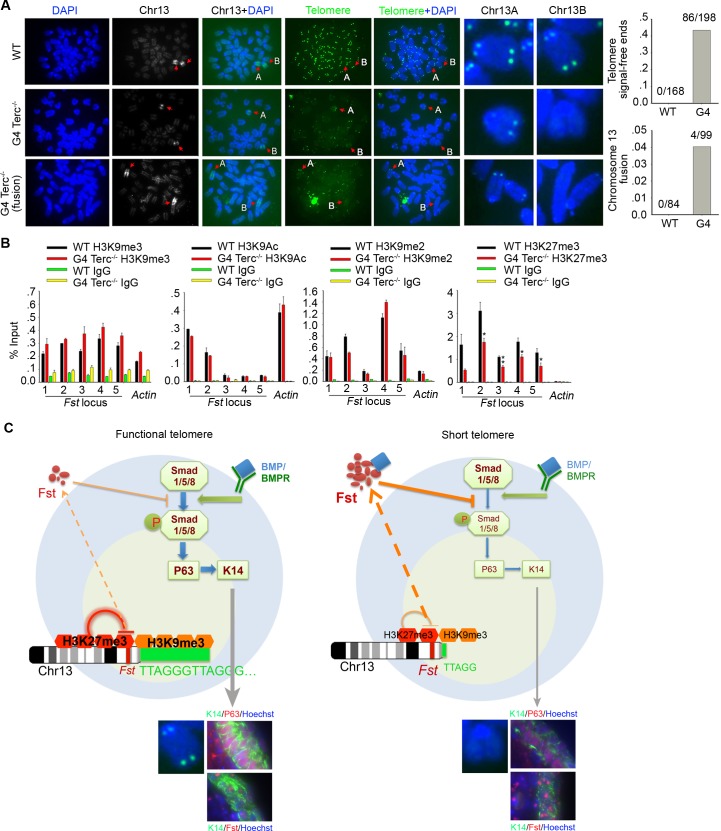
Fig 6. Fst is regulated by epigenetic modification.
(A) Frequency of telomere signal-free ends and fusion of chromosome 13 in G4 Terc–/–ES cells, compared with WT ES cells. Telomere FISH by PNA probe and chromosome identification by XMP13 probe of WT and G4 Terc–/–ES cells. Arrows indicate chromosome 13 stained with XMP13. Chr13A and Chr13B are a pair of chromosome 13 in the same spread. Loss of telomeres near Fst gene locus and fusion of chromosome 13 are compared between WT and G4 Terc–/–ES cells. (B) ChIP-qPCR analysis of abundance of H3K9me3, H3K9me2, H3K9Ac, and H3K27me3 at Fst promoter loci in WT and G4 Terc–/–ES cells. Mean ± SEM (n = 3). *, p<0.05; **, p<0.01, compared to WT cells. (C) A simplified model showing regulation by telomere length of Fst/BMP4-Smad/P63 signaling in epidermal stem cell specification and differentiation. With functional telomeres, enrichment of PRC2/H3K27me3 at Fst promoter foci represses Fst, maintaining normal BMP4-Smad signaling and proper expression levels of P63 and Keratins (e.g. K14), in the specification and differentiation of epidermis and hair follicles. In the event of telomere shortening or loss, abundance of H3K27me3 at Fst foci is reduced, and this causes increased expression of Fst, which impairs BMP4-pSmad signaling, and consequently reduces P63 and K14 expression, epidermal stem cell specification and differentiation.