Figure 3. Mouse models for Prdm14-driven cancer initiation.
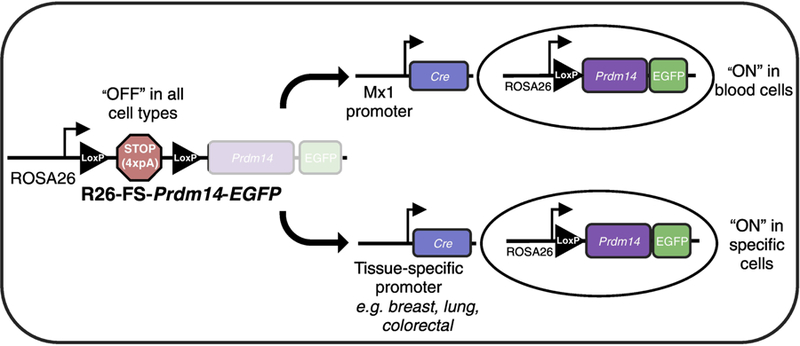
To allow for spatiotemporal control of Prdm14 expression, a mouse line was engineered to carry a transgene inserted downstream of the ubiquitously and constitutively active ROSA26 (R26) promoter. A loxP-STOP-loxP “floxed-STOP” (FS) cassette consisting of a 4X-repeated polyA sequence (pA) lies upstream of the mouse Prdm14 coding sequence, an internal ribosomal entry site and enhanced green fluorescent protein (EGFP). In this configuration, transcription in R26-FS-Prdm14-EGFP mice does not proceed past the STOP cassette and Prdm14 is not expressed. To model an initiating event where Prdm14 becomes aberrantly expressed, R26-FS-Prdm14-EGFP mice are crossed with mice engineered to express the Cre recombinase enzyme under the control of tissue specific promoters. Within a specific cell type, Cre catalyzes site-specific recombination between the loxP sites to excise the STOP cassette and allow for transcription of Prdm14 and EGFP. To study Prdm14-induced leukemogenesis, transgenic mice carrying the Mx1-Cre transgene were mated to mice carrying R26-FS-Prdm14-EGFP. The Mx1 promoter is activated using polyinosinic-polycytidylic acid, leading to the expression of Cre, which deletes the FS cassette, and allows for Prdm14 expression in HSCs. This system can be expanded into other cell types to model many different Prdm14-initiated cancers.
