Fig. 3. Cas mediates mechanical loading–induced suppression of bone resorption and RANKL expression in osteocytes.
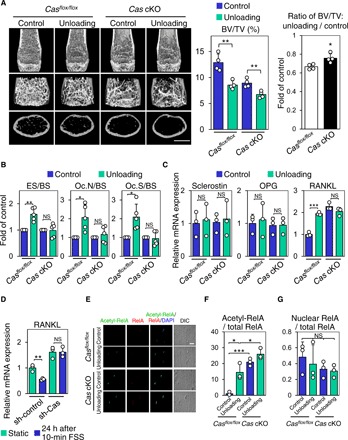
(A) Unloading effect on bone mass in control and Cas cKO mice. Representative μCT images of distal femurs of mice subjected to 2-week hemilateral hindlimb unloading are shown. Scale bars, 1 mm (left). BV/TV was determined as in Fig. 2C (center), and the ratio of unloading to control was calculated in each mouse (right) (n = 4 mice per group). (B) Unaltered bone resorption parameters in unloaded bones of Cas cKO mice. Data from tibiae of mice subjected to 2-week hemilateral hindlimb unloading are shown. Values from unloaded bones were normalized against data from their contralateral controls, which were set at 1 (n = 5 mice per group). (C) Unaltered RANKL expression in osteocytes in unloaded bones of Cas cKO mice. mRNA expression levels of sclerostin, OPG, and RANKL in the osteocyte fractions derived from mice subjected to 1-week hemilateral hindlimb unloading were analyzed as in Fig. 2F. Values were normalized against the control sham–operated hindlimbs (n = 3 mice per group). (D) Loss of FSS effect on RANKL expression in Cas-knockdown MLO-Y4 osteocytes. MLO-Y4 osteocytes with and without Cas knockdown were subjected to FSS experiments as in Fig. 1G. The levels of RANKL mRNA expression were normalized against the static controls, which were set at 1 (n = 3). (E) Loss of mechanical unloading effect on RelA acetylation in osteocytes of Cas cKO mice. Confocal images of anti–acetylated RelA (green), anti-RelA (red), and nuclear (DAPI) staining of osteocytes in midshaft tibiae are shown. Scale bar, 10 μm. (F and G) RelA acetylation (F) and nuclear distribution (G) were quantified as in Fig. 1C with the mean value from control (loaded) bones of control mice (column 1) set at 1 (n = 3 mice per group).
