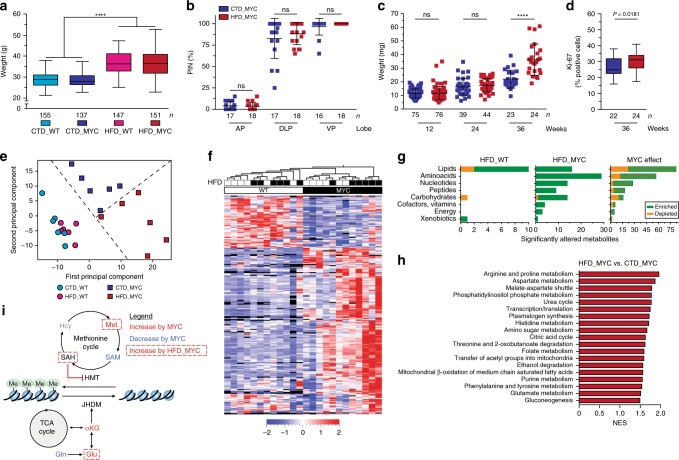
Fig. 1.
High-fat diet reprograms prostate cancer metabolome and accelerates disease progression. a Mice fed a high-fat diet (HFD) develop diet-induced obesity at 12 weeks of age (n = biologically independent animals; two-way ANOVA, median, whiskers ± min/max; ****P < 0.0001). b–d HFD does not alter the penetrance of prostatic intraepithelial neoplasia (PIN) at 12 weeks of age (b, n = biologically independent lobes; unpaired t test, mean ± s.d.; CTD: control diet; AP: anterior prostate; DLP: dorsolateral prostate; VP: ventral prostate; ns: non significant), but does lead to a greater tumour burden (c, n = biologically independent lobes; Welch’s t test, mean ± s.d.; ****P < 0.0001) and to cell proliferation, as assessed by Ki-67 (d, n = biologically independent lobes; unpaired t test, median, whiskers ± min/max) in the VP, at 36 weeks of age. e Principal component analysis identifies a distinct metabolic profile in the VP that is triggered by HFD, in a MYC context (n = 6 biologically independent VP/condition, 414 metabolites detected). f, g Representation of all metabolites significantly altered by HFD in a WT (n = 12) or a MYC (n = 89) context, or by MYC overexpression irrespective of the diet (n = 214) (f, unsupervised hierarchical clustering, P < 0.05 and FDR < 0.15); the breakdown of metabolite classes is shown g. h Metabolite Set Enrichment Analysis (MSEA) revealed metabolic pathways significantly enriched by HFD in MYC-transformed VP (P < 0.05 and FDR < 0.15). i Metabolic rewiring triggered by MYC and by HFD in a MYC context suggests dampened histone methylation. Hcy: homocysteine (undetected); Met: methionine; TCA: tricarboxylic acid (citric acid cycle); Gln: glutamine; Glu: glutamate. Source data are provided as a Source Data file