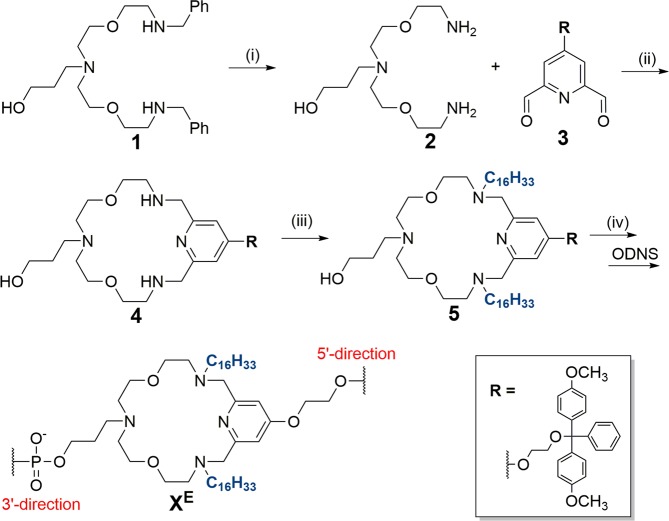
Figure 3.
Synthesis and reaction conditions for compound 4-5a. Synthesis of 1 and 3 are described in ref.37. (i) Pd(OH)2, MeOH, rt (2, 90%). (ii) 1: DMTr protected dialdehyde, CaCl2, anhydrous MeOH, 3Å mol. sieves, reflux; 2: NaBH4, 5 °C → rt (4, 59%). (iii) 1: Hexadecanal, 2: NaBH(OAc)3, anhydrous DCE, 5 °C → rt, 3Å mol. sieves (5, 50%). (iv) 2-Cyanoethyl-N,N-diisopropylchlorophosphoramidite, anhydrous DCE, 5 °C → rt (5a, 80%). 5a incorporated of into oligonucleotides = XE.