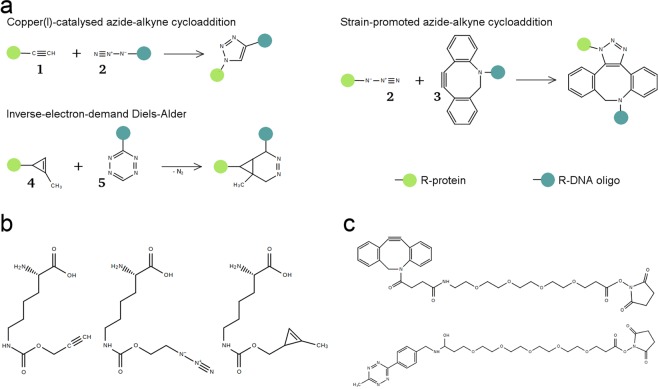
Figure 2.
Overview of the bio-orthogonal reactions, unnatural amino acids, and DNA modifications employed in this study. (a) Terminal alkynes (1) and azides (2) can form 1,4-disubstituted 1,2,3-triazoles in a 1,2-dipolar cycloaddition catalysed by copper. Azides can also react with strained alkynes such as DIBO/DBCO (3) in strain-promoted azide-alkyne cycloadditions that do not require a catalyst. Cyclopropenes (4) react with tetrazines (5) in inverse-electron-demand Diels-Alder reactions to diazanorcaradienes. (b) Lysine derivatives bearing alkyne, azide and cyclopropene moeities were introduced into PR65. (c) DNA oligonucleotides were modified with DBCO and tetrazine functional groups.