Fig. 6.
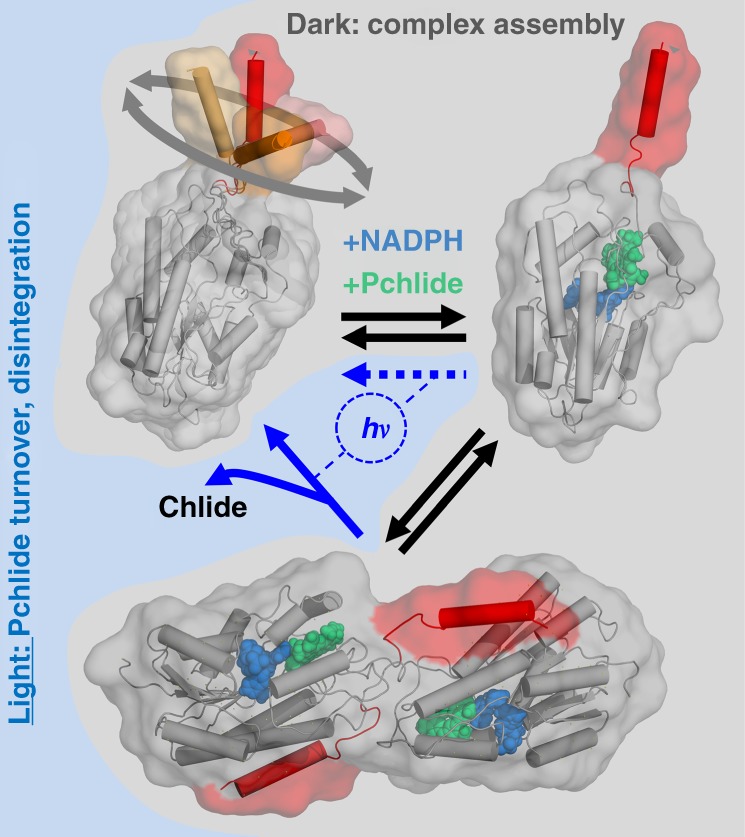
Model illustrating the proposed structural changes occurring in TeLPOR due to holoprotein formation and light-dependent Pchlide turnover. For a detailed description of the model refer to the main text. Without Pchlide and NADPH, TeLPOR is monomeric, with the C-terminal α-helical extension moving freely (illustrated by four different helical conformations extracted from the corresponding MD trajectory; orange to red cartoon). Assembly of the holoprotein complex in the dark might result in rigidification of the protein, which in turn allows for dimerization, mediated by the C-terminal extension, the Q-axis interface and the active site surface patch. Light-dependent conversion of Pchlide to Chlide, followed by product release would then trigger the dissociation of the dimer. The dashed blue arrow indicates the possibility that also the monomeric holoprotein shows light-dependent activity, as inferred by MWA-AUC. Pchlide and NADPH are shown as green and blue spheres, respectively. The molecular surface of the models are shown as transparent grey surface
