FIG 2.
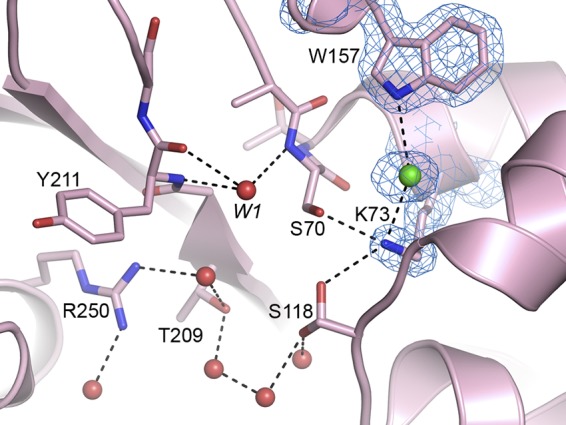
Final 2Fo − Fc density map (blue mesh, 1.0σ) for the Lys73 and Trp157 residues in apo–OXA-48 (pink ribbons). Residues involved in substrate binding and catalysis are shown as pink sticks, and their hydrogen-bonding networks are indicated by dashed lines. The chloride anion in the deacylation water pocket is shown as a green sphere in the 2Fo − Fc density, and the interactions that it makes with the lysine and tryptophan are shown. Water molecules that hydrogen bond to the active-site residues are shown as red spheres. Water molecule W1 is located in the oxyanion hole between Ser70 and the main chain of Tyr211.
