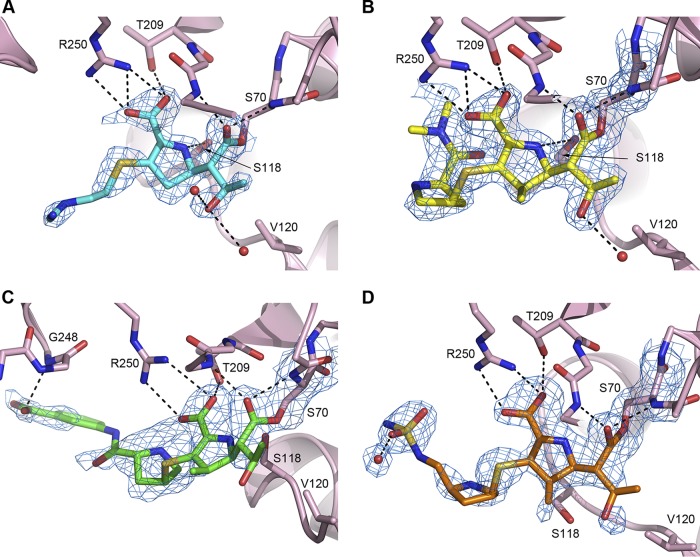
FIG 3.
Final refined 2Fo − Fc electron density maps (blue mesh, 1.0 σ) for the carbapenems imipenem (A), meropenem (B), ertapenem (C), and doripenem (D) bound to OXA-48. The hydrogen-bonding interactions anchoring the four carbapenems in the active site of the enzyme are indicated by dashed black lines. In panels A and B, the Ser118 side chain is partially occluded behind the substrate and indicated by an arrow. Water molecules that form hydrogen-bonding interactions with the substrates are shown as small red spheres. In panel C the orientation of the complex is slightly different from that in the other three panels to show the hydrogen-bonding interaction between the ertapenem tail and Gly248 clearly. In panel D, the difference in the location of the Ser118 residue is due to a conformational difference in the loop containing Ser118 and Val120 in one of the molecules in the asymmetric unit.