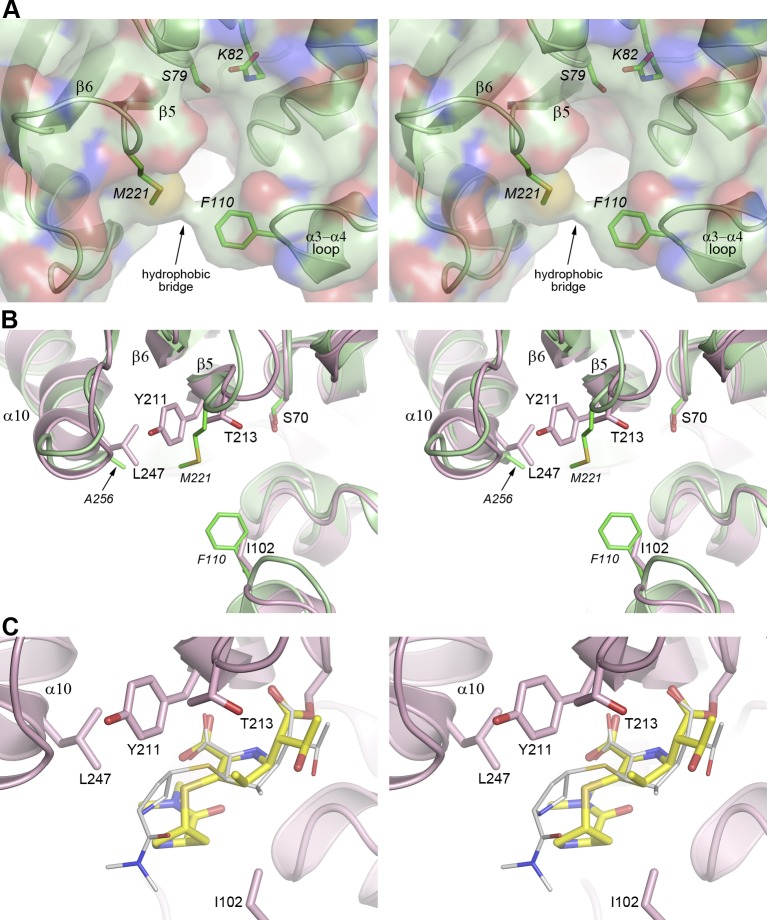
FIG 5.
Stereoviews of OXA-23 and OXA-48. (A) The active site of OXA-23 (green ribbons and sticks) showing the hydrophobic bridge between Phe110 and Met221. The solvent-accessible surface of the enzyme is also shown partially transparent, and the hydrophobic contact between these two residues is evident. (B) Superposition of OXA-23 and OXA-48. The residues lining the active site are shown with the β-strand side at the top and the helical domain side at the bottom. The OXA-48 molecule is colored pink, and OXA-23 is colored green. (C) The OXA-48–meropenem complex (pink ribbons) showing the bound meropenem as the Δ2 tautomer in yellow. The Δ1R meropenem tautomer from the bridge-deficient OXA-23 mutant complex is shown as thin gray sticks.