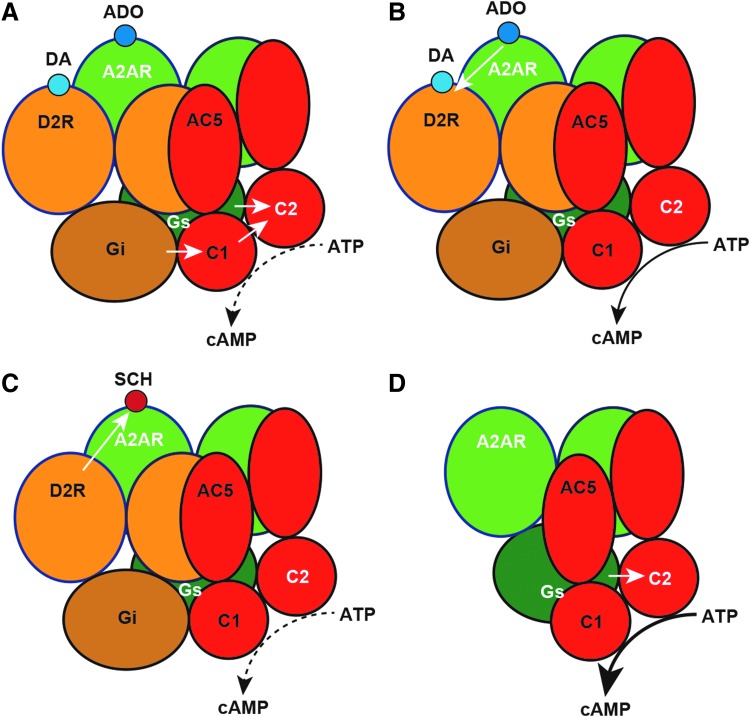
FIG. 1.
Functional and pharmacological properties of the A2AR-D2R heterotetramer. (A) Canonical interaction, by which a D2R agonist, such as DA, counteracts the effect of an A2AR agonist, such as ADO, by a Gs-Gi antagonistic interaction at the AC level (subtype AC5). (B) Allosteric interaction, by which A2AR ligands antagonistically counteract the affinity and efficacy of D2R ligands. (C) Ligand-independent changes in the properties of A2AR ligands on heteromerization with the D2R, such as the selective decrease in the affinity of the A2AR antagonist SCH442416. (D) Increased A2AR signaling in the absence of the D2R and in the absence of A2AR ligands (constitutive activity) by the A2AR homomer. C1 and C2, catalytic domains of AC5. White arrows indicate the direction of the intermolecular interaction. Black arrows indicate the intensity of cAMP formation (from broken to small and large solid arrows). A2AR, A2A receptors; AC, adenylyl cyclase; ADO, adenosine; D2R, D2 receptor; DA, dopamine.