Figure 3.
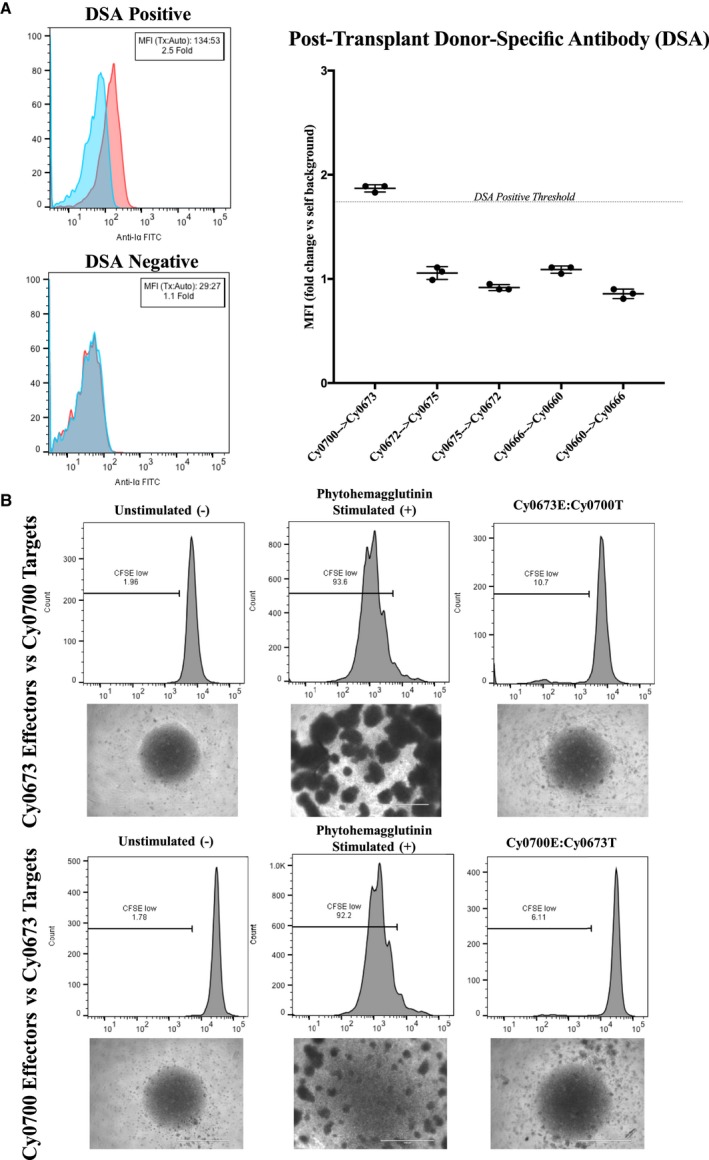
Assessment of de novo donor‐specific antibody (DSA) development and mixed lymphocyte reactions. Blood serum samples were drawn from animals pretransplant and posttransplant to assess for the presence of preexisting and de novo DSAs, respectively. Serum was incubated with autologous or transplant donor peripheral blood mononuclear cells (PBMCs), washed, and then stained with secondary antimacaque IgG–fluorescein isothiocyanate antibody to detect bound DSA (n=3 replicates per sample). A, Left, Representative flow cytometric staining of a DSA‐positive transplant recipient (Cy0700) shows a shift in median fluorescence intensity (MFI) >1.75× over autologous background. Data are representative of n=5 repeated experiments. Right, Representative graph summarizing the results of one experiment, including DSA‐positive and DSA‐negative animals. B, One‐way mixed lymphocyte reactions. Top, Target PBMCs from transplant donor Cy0700 were irradiated (30 Gy) and labeled with CellTrace‐Violet dye, whereas effector PBMCs from transplant recipient Cy0673 were not irradiated and labeled with CFSE (carboxyfluorescein succinimidyl ester) proliferation dye. Images of culture wells were taken showing the unstimulated effector PBMCs, those effectors stimulated with T‐cell mitogen phytohemagglutinin, and the cocultured effectors/targets. Bottom, Target and effector were switched for the same transplant pair and analyzed as above. Data are representative of triplicate wells per condition.
