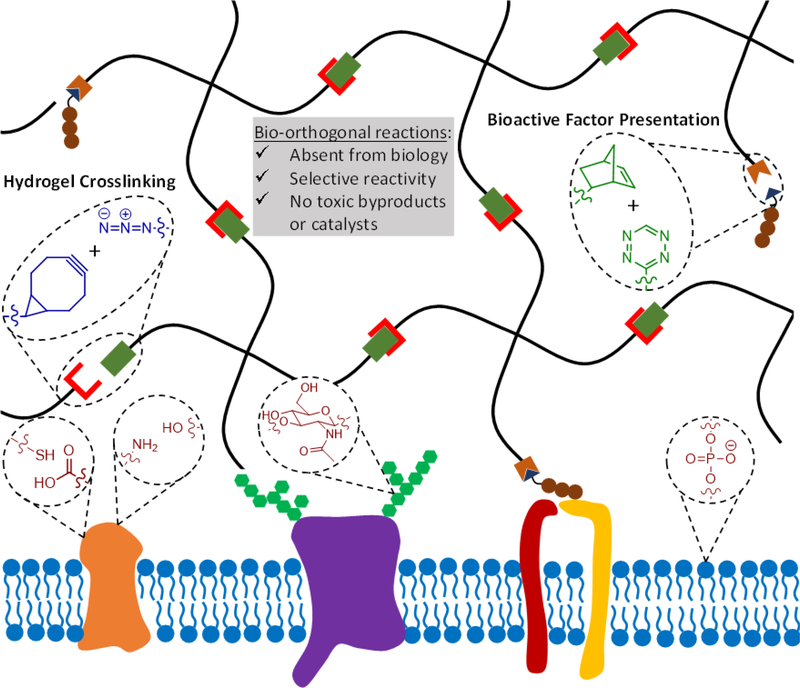
Figure 1.
Bio-orthogonal reactions make use of reactive groups that do not naturally occur in biological systems, react selectively to avoid cross-reactivity with various biological functional groups, and do not produce toxic byproducts or require toxic catalysis. In the context of hydrogels as engineered ECMs, bio-orthogonal chemistries can be used to crosslink the hydrogel and modulate the presentation of biochemical signals. Two example bio-orthogonal reactions, strain-promoted azide-alkyne cycloaddition (SPAAC) in blue and tetrazine-mediated inverse-electron demand Diels-Alder (IED-DA) reaction in green, are depicted to form a hydrogel (black network) adjacent to the cell surface (blue lipid bilayer). Common biological functional groups present on the cell surface that may be targets of cross-reactivity in non-bio-orthogonal reactions are also depicted in red.