Table 1.
Bio-orthogonal chemistries to crosslink hydrogels.
| Gelation Chemistry | Reactive Group #1 | Reactive Group #2 | Reaction Product | Notes | References |
|---|---|---|---|---|---|
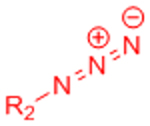
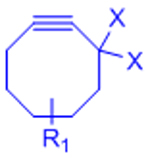
| Copper-catalyzed azide-alkyne cycloaddition (CuAAC) | 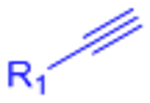 |
 |
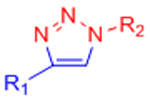 |
Potential Cu2+ toxicity may necessitate use of chelating ligands | [24] |
 |
 |
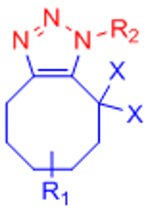 |
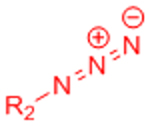
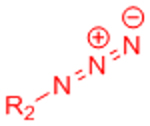
X: H (Slow reaction kinetics) X: F (Improved kinetics) | [25, 27–30, 123, 124] | |
| Strain-promoted azide-alkyne cycloaddition (SPAAC) | 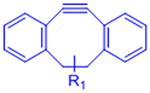 |
 |
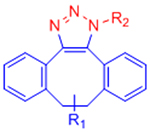 |
[31–33] | |
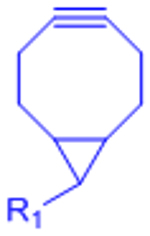 |
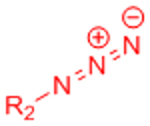 |
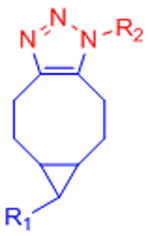 |
[34–37, 162] | ||
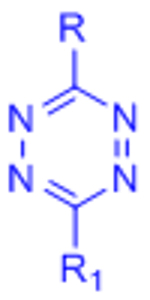
| Inverse electron demand Diels-Alder (IED-DA) | 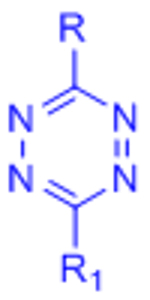 |
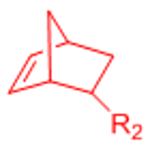 |
 |
[51–54, 132] | |
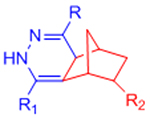
 |
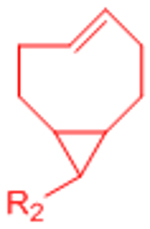 |
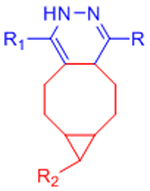 |
Extremely rapid gelation kinetics | [56] | |
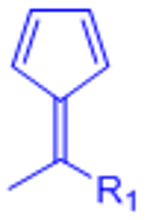
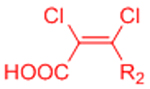
| Diels-Alder (DA) | 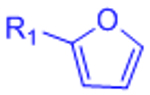 |
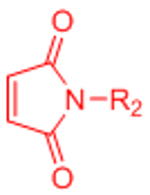 |
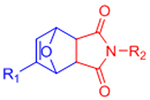 |
Maleimides can cross-react with thiols | [58–68, 70, 71] |
 |
 |
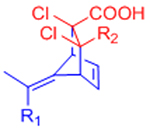 |
Reversible under physiological conditions | [74] | |
| Staudinger ligation | 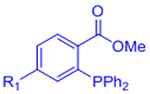 |
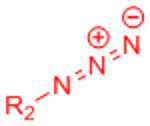 |
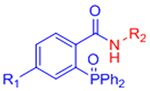 |
Slow reaction kinetics | [35, 75, 76] |
| Nitrile Oxide Cycloaddition | 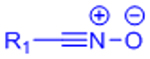 |
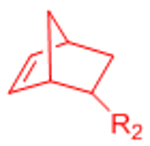 |
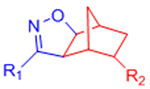 |
Highly reactive nitrile oxide must be generated in situ | [78, 79] |
