Abstract
Patient: Male, 66
Final Diagnosis: Enthesitis/gonarthritis
Symptoms: Arthralgia
Medication: —
Clinical Procedure: —
Specialty: Immunology
Objective:
Unusual clinical course
Background:
Recent discoveries in the field of immunometabolism, and on the role of the serine-threonine kinase mTOR as a sensor of nutrients, integrator of cellular signaling pathways, and regulator of metabolism, have widened our understanding of the connection between nutrition, health, and diseases. Epidemiological studies have shown that higher sugar-sweetened beverage consumption is associated with increased risk of developing chronic diseases, including cardiovascular disease, type 2 diabetes mellitus, obesity, non-alcoholic fatty liver disease, gout, and rheumatoid arthritis and to worse symptoms in some patients with rheumatoid arthritis. Anabolic metabolism has been demonstrated to favor the differentiation of proinflammatory T lymphocytes while katabolic metabolism to favor regulatory T lymphocyte differentiation.
Case Report:
In a 66-year old male, the onset of gonarthritis and enthesitis and worsening of these symptoms 3 months later were associated with excessive intake of desserts. Two weeks after starting strict avoidance of sugar containing nutrients and beverages symptoms disappeared. During the next 6 months, on 3 occasions, the exceptional consumption of a dessert was followed by a mild and transient recurrence of the symptoms.
Conclusions:
The repeatedly observed recurrence of enthesitis/arthritis symptoms following sugar intake and its disappearance following avoidance of sugar, represents an extreme example of a link between metabolism and local inflammation in the reported individual. The rapid absorption of the monosaccharides glucose and fructose from the intestine, where they derive from hydrolysis of the disaccharide sucrose (sugar) might lead to overactivation of mTOR if not counterbalanced by other mTOR interfering mechanisms.
MeSH Keywords: Arthritis; Autoimmunity; Dietary Sucrose; T-Lymphocytes, Regulatory; TOR Serine-Threonine Kinases
Background
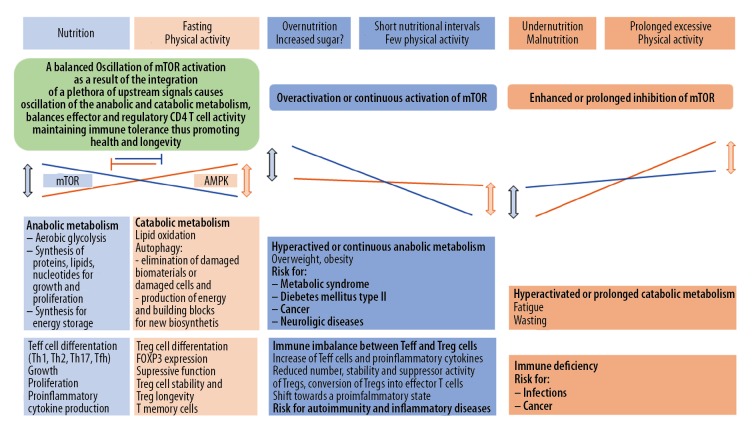
Living organisms have to adapt to the environment and nutrients availability. A key role in this adaptation is played by the evolutionary conserved enzyme mechanistic (prior mammalian) target of rapamycin (mTOR), a threonine-serine protein kinase [1,2]. Sabatini provides this description: “mTOR, as the catalytic subunit of 2 distinct protein complexes, mTORC1 and mTORC2, is the major regulator of growth in animals and controls most anabolic and catabolic processes in response to nutrients and nutrient-induced signals like insulin” [2]. Hence mTORC1 regulates metabolism and growth and is regulated by nutrients. I addition, mTOR represents a central node of the cellular signaling network, a master regulator, that senses nutrients and integrates a plethora of other upstream signals into downstream metabolic programming and reprogramming. It drives the physiological oscillation of anabolic processes, necessary for growth, proliferation, production of functional proteins and storage of energy fuels, and oscillation of catabolic processes including autophagy, necessary for energy production during fasting, degradation of damaged cells or its components, and provision of building blocks for new biosynthesis. Activation of mTOR induces anabolic processes and inhibits catabolic processes including autophagy and vice versa [1–6]. There are 26 proteins that comprise the nutrient sensing arm of the mTOR pathway that have so far been identified reflecting a significant amount of protein space regulating mTOR via nutrients [2].
Under starvation, the levels of nutrients and growth factors drop, inducing a catabolic state in which energy stores are mobilized to maintain essential functions. Meanwhile, it is known that mTORC1 is inhibited under starving conditions. Mice that have genetically manipulated continuously active Rag GTPases, and as a consequence have continuously active mTOR, once born and separated from the maternal supply of nutrients do not survive periods of fasting because they cannot switch from an anabolic to a catabolic state [3]. In addition to a crucial role for mTOR in physiology, metabolism and the aging process, it is known to be deregulated in common diseases [1–5]. Mechanistical studies using cell or animal models with either enhanced or inhibited mTOR activation suggest analogous mechanisms may occur in overnutrition or prolonged starvation. Importantly, mTOR hyperactivation from genetic or dietary manipulation has been shown to result in insulin resistance and impaired glucose homeostasis [3]. Likewise, there are reports linking mTOR dysregulation (hyperactivation) caused by overnutrition and/or genetic mutations to common diseases such as metabolic syndrome, diabetes mellitus, cancer, and neurologic diseases [3–5].
The term mosaic of autoimmunity, coined by Yehuda Shoenfeld, alludes to the many genetic, environmental, and behavioral factors potentially contributing to autoimmunity and autoimmune diseases [7]. Distinguishing self and non-self is a complex and dynamic process. Because central immune tolerance due to negative selection in the thymus is incomplete, it has to be complemented by peripheral tolerance exerted through specific suppression by the regulatory T cells (Tregs) [8]. Insufficient Treg cell function is considered a main cause of autoimmunity [9]. Following specific T cell receptor stimulation, naïve CD4 T cells differentiate into effector T cells: T helper 1 (TH1), T helper 2 (TH2), T follicular helper (TfH), T helper 17 (TH17), or T regulatory cells (Treg). T cell differentiation depends on the intensity and duration of the specific T cell receptor stimulation, costimulatory signals, cytokines, and as a more recent discovery, also on metabolic pathways driven by mTOR [10–12]. Metabolic signaling has emerged not only as an activation signal, but also as one that can influence and shape differentiation. The mTOR signaling pathway has an indispensable role in the process of T cell fate determination, including the differentiation of naïve T cells into either effector or regulatory T cells and the development of CD8 memory T cells. Inhibition of mTOR with rapamycin has been shown to facilitate the induction of anergic and regulatory CD4+ T cells – 2 crucial components of peripheral tolerance – as well as the differentiation of memory CD8+ T cells. Over-activation of mTOR in Treg cells was found to reduce their suppressive activity in a T cell-mediated mouse colitis model. Furthermore, in inflammatory conditions, over-activation of mTOR promoted Treg instability, loss of FOXP3 expression, and conversion to effector T cells that produced proinflammatory cytokines, (e.g., IL-17 and IL-1β) leading to the loss of suppressive function [10–12]. Considering these discoveries, it has been suggested that increased metabolic pressure (overnutrition) might contribute to breach immunological self-tolerance [13,14] (Figure 1).
Figure 1.
Hypothetical simplified representation of mTOR regulating cell metabolism, growth, proliferation, and immune function in health and disease. Teff – effector cell; Treg – regulatory T cell; Th1 – helper1 T cell; Th2 – helper2 T cell; Th17 – helper17 T cell; Tfh –follicular helper T cell; AMPK – adenosine monophosphate activated protein kinase; FOXP3 – forkhead box protein 3. Blue areas – mTOR activation; orange areas – mTOR inhibition.
Observational data suggest that higher sugar-sweetened beverage consumption is linked to a host of chronic diseases, including cardiovascular disease, type 2 diabetes mellitus, obesity, non-alcoholic fatty liver disease, and gout [15]. A study of 2 large prospective cohorts comprising 79 570 and 107 570 young and middle-aged women, each with >2 decades of follow-up, revealed that regular consumption of sugar-sweetened soda was associated with increased risk of seropositive rheumatoid arthritis (RA), independent of other dietary and lifestyle factors [16]. In a diet survey on 217 people with RA 24% of them reported that foods affect their RA symptoms. Soda with sugar (12.7%) and desserts (12.4%) were most often reported to worsen RA symptoms among those who consumed them [17]. Another study of 39 345 middle-aged women who had completed a 131-item validated, semiquantitative food-frequency questionnaire, revealed that a high intake of rapidly digested and absorbed carbohydrates was significantly and positively associated with plasma highly sensitive C-reactive protein, independent of conventional risk factors for ischemic heart disease, thereby increasing the risk of ischemic heart disease, especially in overweight women [18].
Case Report
A healthy 66-year old male, a retired hospital physician and passionate hiker, was affected by an enthesitis of the mesial insertion of the musculus quadriceps and an ipsilateral gonarthritis in May 2017. Since his retirement approximately 1-year before the reported episode, he used to hike up to the top of his “house” mountain (altitude difference over 1000 meters) at least once a week and to ran down afterwards. He had done so also on the day before his complaints began. His height was 172 cm, weight 75 kg, calculated body mass index (BMI) was 25.4 kg/m2. In the past 30 years he had experienced several episodes of mild enthesitis and/or arthritis at different sites (toe, foot, knee, hip, shoulder, finger). These episodes had disappeared spontaneously and without treatment within weeks or months. His family history was remarkable in that his father, one cousin on the father’s side, and one nephew had been affected by autoimmune thyroiditis. Routine medication consisted of valsartan 160 mg daily that the patient had been taking for 15 years. Blood pressure was 130/85 mm Hg. Routine blood examinations including red and white blood cell count, liver and kidney function tests, urinalysis, glycemia, blood sedimentation rate, C reactive protein (0.15–0.34 mg/dL), had been repeatedly within the reference range except for an elevation of high-density lipoprotein cholesterol (HDL) (133–186 mg/dL [reference <115 mg/dL]). HLA-B27, rheumatoid factor, HIV, and Borrelia burgdorferi antibody tests were negative.
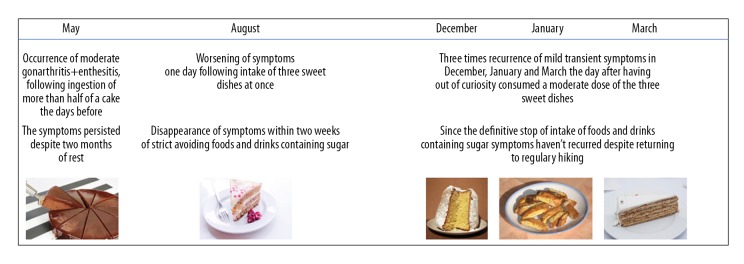
The patient reported a burning pain of the knee disturbing his sleep and decreasing with movement. Furthermore, he felt pain at the tendon insertion site occurring especially when walking downwards, while walking upwards or riding a bicycle were not painful. Therefore, he avoided going downwards for the following 2 months, but without improvement. One day the complaints had worsened. Therefore, he thought about events that might have triggered the aggravation the symptoms. The only extraordinary event the patient recalled was an exceptional consumption of 3 sweet dishes the day before. Moreover, he remembered that in the 2 days prior to the initial onset of the knee symptoms in May he had eaten more than the half of his birthday cake, which he had never done before. Therefore, he decided to avoid dishes and drinks containing sugar, and within 2 weeks his complaints disappeared. Out of curiosity, and because he was not convinced of a causal relationship between intake of sweets and his joint complaints, the patient exceptionally started to have sweet dishes again. Over the next 6 months this happened 3 times and each time was followed by transient recurrence of the knee complaints, although with weakened intensity (Figure 2). Since then, he definitively avoided food and beverages containing sugar. Moreover, he regularly hiked in the mountains without complaints. Avoiding sugar also has led to a 3 kg weight reduction and to a BMI of 24.3 kg/m2.
Figure 2.
Timeline of the enthesitis/gonarthritis symptoms associated with the consumption of sweet dishes. The images of the sweet dishes does not correspond to those ingested nut to similar ones.
Discussion
Glucose derived from digestion of carbohydrates is vital for any organismal cell. However, the dose of glucose does matter. An increased dose of glucose due to persistent overnutrition or due to the rapid absorption of glucose derived from rapid enzymatic scission of the disaccharide sucrose (common sugar) into glucose and fructose in the intestine may lead to over-activation of the mTOR signaling pathway. Overactivation of mTOR, if not counterbalanced by inhibitory mechanisms, may have unfavorable consequences for health [3–5]. We wonder whether in the participants of the mentioned epidemiological studies [15–18] and in the described case, the increased intake of sugar, which most likely had been rapidly absorbed, could have led to an over-activation of the mTOR signaling pathway favoring glycolysis, increasing effector T cell cytokine production and proliferation as well as promoting the differentiation of naive T cells into TH17 cells (actors in inflammation/autoimmunity) at the expense of Treg cells (actors in immunotolerance). Might such a shift of the balance between effector T cells and regulatory T cells, towards effector T cells be at the basis of a shift towards a proinflammatory state and/or autoimmunity? Interestingly, in the described case the excessive consumption of sweets was followed by the onset of enthesitis and gonarthritis and the less excessive consumption of sweets 3 months later was followed by worsening of the symptoms. While in 3 occasions even the ingestion of a single sweet, considered as a “normal dose”, was followed by symptom recurrence, only strict avoidance of sugar-sweetened food and beverages resulted in complete resolution of the symptoms (Figure 2). The fact that the enthesitis/gonarthritis symptoms could be repeatedly triggered by sugar-containing sweets appears to be strong evidence in favor of a causal relationship between the inflammatory symptoms and sugar intake. We speculate whether the excessive sugar consumption in the first 2 occasions and the consequently rapid glucose uptake in T cells had enhanced the function and induced the proliferation of autoreactive effector T cells via over-activation of mTOR. Furthermore, the consumption of a moderate dose of sugar in the next 3 occasions might have been sufficient to enhance the effector T cell function of a previously increased number of autoreactive T cells, and to weaken Treg cell function causing the mild and transient recurrence of the symptoms. One may hypothesize that running down a mountain in the described individual’s case might have caused microlesions to the knee, that usually are repaired by a physiological asymptomatic, transient inflammatory process. However, in this case this coincided with excessive sugar intake which we believe resulted in an over-activation of mTOR at the molecular level. This over-activation might have shifted the cellular balance towards a proinflammatory state and weakened Treg suppressive function causing gonarthritis and enthesitis (Figure 1).
Obviously, such a causal relation is not true for most people who consume sugar. The term mosaic of autoimmunity, coined by Yehuda Shoenfeld, allude to the many factors contributing to pathological autoimmunity [7]. Every individual affected by autoimmune diseases might have their own mosaic of contributing factors. In some individuals excessive sugar intake might be a piece of their mosaic of contributing factors, and in certain circumstances, might function as a trigger of pathological autoimmunity as was probably repeatedly the case in the described patient. In this patient the enthesitis/arthritis pain recurred following sugar consumption and disappeared after strict avoidance of sugar in 4 occasions (Figure 2).
The positive family history for autoimmune thyroiditis allude to a possible genetic predisposition for autoimmune diseases. It is thought that genetic predisposition is a prerequisite for most autoimmune diseases [8]. A body mass index of 25.4 kg/m2 despite regular physical activity (hiking in the mountains) indicates overnutrition and, hence, additional metabolic pressure as well as increased production of adipokines (leptin) might have contributed to a proinflammatory state. Leptin, the adipose tissue derived hormone, is known to regulate T cell proliferation and cytokine production and to contribute to activation of the mTOR pathway in Treg cells [6,11,13,14]. Leptin concentration is proportional to fat mass and is positively associated with overweight and increased susceptibility to chronic inflammation and autoimmune diseases. Leptin or leptin receptor knock out mice display resistance to autoimmune disorders including experimental autoimmune encephalomyelitis, experimental colitis, Ag-induced arthritis, and type 1 diabetes mellitus [19]. Little is known about the effect of food containing sugar or other high refined carbohydrates on gut microbiota, which in turn influence the immune system.
The recent discoveries of the molecular mechanisms and signaling pathways upstream and downstream of mTOR that drive the oscillation of anabolic and catabolic metabolism and determined differentiation and function of T cells, might contribute to the understanding the described case and the reported epidemiological observations. Thus, this suggests a potential causal association between increased sugar intake and inflammatory or autoimmune reactions, at least in individuals with hypothetic deficits of the various mTOR antagonizing molecular mechanisms. Because of the complexity of the immune system regulation including the induction and maintenance of immune tolerance by Treg cells, it may be difficult to prove such a hypothesis. Nevertheless, the scientific insights on the central regulatory role of the mTOR pathway regarding cell metabolism and its nutrient dependent activation as well as its known impact on T cell differentiation, proliferation, and function suggest a direct link between overnutrition and inflammatory states. This is strengthened by results of epidemiological studies indicating that reduction of sugar intake could have a preventive effect on the risk for autoimmune and other inflammatory diseases and could contribute to clinical improvement in some patients [15–18].
Conclusions
Avoiding excessive sugar intake and overnutrition and favoring the physiological oscillation between anabolic and catabolic metabolism due to physical activity and/or fasting might promote health and contribute to the prevention of autoimmune and other diseases at least in genetically predisposed individuals. Individuals can try to avoid sugar intake and/or overnutrition, and try to exert more physical activity and/or fasting and observe if this is followed by short-term health benefits. The long-term benefits of such behavior regarding health and prevention of diseases have, for a long time, been widely accepted and demonstrated by epidemiological and interventional studies.
Footnotes
Conflict of interest
None.
References:
- 1.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–22. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabatini DM. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc Natl Acad Sci USA. 2017;114:11818–25. doi: 10.1073/pnas.1716173114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efeyan A, Zoncu R, Chang S, et al. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–83. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zoncu R, Efeyan A, Sabatini DM. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell. 2017;169:361–71. doi: 10.1016/j.cell.2017.03.035. [DOI] [PubMed] [Google Scholar]
- 6.Procaccini C, De Rosa V, Galgani M, et al. An oscillatory switch in mTOR kinase activity sets regulatory T cell responsiveness. Immunity. 2010;33:929–41. doi: 10.1016/j.immuni.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shoenfeld Y, Isenberg DA. The mosaic of autoimmunity. Immunol Today. 1989;10:123–26. doi: 10.1016/0167-5699(89)90245-4. [DOI] [PubMed] [Google Scholar]
- 8.Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017;18:716–24. doi: 10.1038/ni.3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu L, Barbi J, Pan F. The regulation of immune tolerance by FOXP3. Nat Rev Immunol. 2017;17:703–17. doi: 10.1038/nri.2017.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell JD, Pollizzi KN, Heikamp EB, Horton MR. Regulation of immune responses by mTOR. Annu Rev Immunol. 2012;30:39–68. doi: 10.1146/annurev-immunol-020711-075024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi H. Regulation and function of mTOR signaling in T cell fate decisions. Nat Rev Immunol. 2012;12:325–38. doi: 10.1038/nri3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zeng H, Chi H. mTOR signaling in the differentiation and function of regulatory and effector T cells. Curr Opin Immunol. 2017;46:103–11. doi: 10.1016/j.coi.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Procaccini C, Galgani M, De Rosa V, Matarese G. Intracellular metabolic pathways control immune tolerance. Trends Immunol. 2012;33:1–7. doi: 10.1016/j.it.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 14.De Rosa V, La Cava A, Matarese G. Metabolic pressure and the breach of immunological self-tolerance. Nat Immunol. 2017;18:1190–96. doi: 10.1038/ni.3851. [DOI] [PubMed] [Google Scholar]
- 15.Haslam DE, McKeown NM, Herman MA, et al. Interactions between genetics and sugar-sweetened beverage consumption on health outcomes: A review of gene-diet interaction studies. Front Endocrinol (Lausanne) 2018;8:368. doi: 10.3389/fendo.2017.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu Y, Costenbader KH, Gao X, et al. Sugar-sweetened soda consumption and risk of developing rheumatoid arthritis in women. Am J Clin Nutr. 2014;100:959–67. doi: 10.3945/ajcn.114.086918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tedeschi SK, Frits M, Cui J, et al. Diet and rheumatoid arthritis symptoms: Survey results from a rheumatoid arthritis registry. Arthritis Care Res (Hoboken) 2017;69:1920–25. doi: 10.1002/acr.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu S, Manson JE, Buring JE, et al. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am J Clin Nutr. 2002;75:492–98. doi: 10.1093/ajcn/75.3.492. [DOI] [PubMed] [Google Scholar]
- 19.Galgani M, Procaccini C, De Rosa V, et al. Leptin modulates the survival of autoreactive CD4+ T cells through the nutrient/energy-sensing mammalian target of rapamycin signaling pathway. J Immunol. 2010;185:7474–79. doi: 10.4049/jimmunol.1001674. [DOI] [PubMed] [Google Scholar]