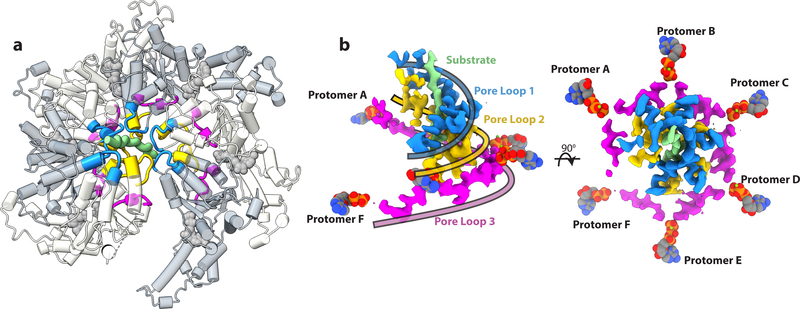
Figure 2. A double-helical staircase of pore loops surrounds the substrate and is coupled by a third pore loop spiral to the ATP binding site.
(a) The three solvent exposed pore loops highlighted on the spastin hexamer structure. Pore loop 1 is colored in blue, pore loop 2 in gold, and pore loop 3 in magenta. (b) Side (Left) and top (Right) view of the EM density of the triple spiral staircase generated by pore loops 1, 2 and 3. Pore loops 1 and pore loops 2 form a tight double spiral that engages to the peptide substrate shown in light green. Pore loops 3 form a shallow spiral between pore loops 1–2 and the nucleotide. Ribbons colored as their corresponding pore loops are overlaid on the EM map to highlight the trajectory of the three pore loop spirals.