Abstract
Background
Metabolic syndrome (MetSyn) is an important risk factor for cardiovascular diseases and type 2 diabetes. It is unknown whether the MetSyn prevalence differs within a homogenous population residing in different settings in Africa and Europe. We therefore assessed the prevalence of MetSyn among Ghanaians living in rural- and urban-Ghana and Ghanaian migrants living in Europe.
Methods
We used data from the cross-sectional multi-centre RODAM study that was conducted among Ghanaian adults aged 25–70 years residing in rural- and urban-Ghana and in London, Amsterdam and Berlin (n = 5659). MetSyn was defined according to the 2009 harmonized definition. Geographical locations were compared using age-standardized prevalence rates, and prevalence ratios (PRs), adjusted for age, education, physical activity, and smoking and stratified for sex.
Results
In men, the age-standardized prevalence of MetSyn was 8.3% in rural Ghana and showed a positive gradient through urban Ghana (23.6%, adjusted PR = 1.85, 95% confidence interval 1.17–2.92) to Europe, with the highest prevalence in Amsterdam (31.4%; PR = 4.45, 2.94–6.75). In women, there was a rural-to-urban gradient in age-standardized MetSyn prevalence (rural Ghana 25%, urban Ghana 34.4%, PR = 1.38, 1.13–1.68), but small differences in MetSyn prevalence between urban-Ghanaian and European-Ghanaian women (Amsterdam 38.4%; London 38.2%).
Conclusion
MetSyn is highly prevalent in Ghana as well as in Ghanaian migrants in Europe. To assist prevention efforts, further research is needed to understand the mechanisms driving the geographical differences in MetSyn prevalence between migrant and non-migrant Ghanaians.
Introduction
Metabolic syndrome (MetSyn) is a collection of five cardiometabolic risk factors including an increased waist circumference (WC), elevated triglyceride levels, lowered high-density lipoprotein cholesterol (HDL-C), elevated blood pressure (BP) and raised fasting plasma glucose (FPG).1 Compared with individuals without the syndrome, individuals with MetSyn have a 3- to 5-fold increased risk for developing type 2 diabetes (T2D)2,3 and a doubled risk for cardiovascular disease (CVD) events.4 Studies on MetSyn in sub-Saharan African (SSA) countries are scarce, but suggest that MetSyn is of importance in this region, with prevalence rates varying from 13.7% in rural Uganda5 to 21% in urban Ghana6 and 34.6% in urban Kenya.7 Rapid urbanization associated with lifestyle changes have been suggested as a major contributor to this rural–urban difference.8 Data on MetSyn prevalence among populations with SSA origin in high-income countries are also limited and show substantial differences in MetSyn prevalence, depending on sex and geographical location of residence.9,10 There is no clear explanation for these differences in MetSyn prevalence, but this might be due to differential exposures in the countries of settlement, leading to variation in distribution and clustering of MetSyn components.11 As MetSyn is an important risk factor for CVD and T2D, it is important to obtain more insight into the distribution of MetSyn components among SSA populations and how this varies between populations from SSA origins in different settings. Therefore, the aim of this study was to assess whether the prevalence of MetSyn and its individual components differ among Ghanaian migrants in urban areas in The Netherlands, UK and Germany and in their counterparts living in rural and urban Ghana.
Methods
The rationale, conceptual framework, design and methodology of the RODAM study have previously been described elsewhere,12 and will be summarized here.
Study population and study design
The RODAM study, a multi-centre, cross-sectional study, was carried out between 2012 and 2015, and included Ghanaians aged ≥25 years living in rural and urban Ghana, and in the cities of London, Amsterdam and Berlin. Ghanaians are one of the largest SSA migrant groups in the UK, Netherlands and Germany, of whom the majority resides in their respective capital cities.13–15 Various recruitment strategies were necessary at the different study sites, due to differences in population registration systems across European countries and in Ghana. In brief, in a random sampling procedure, participants were drawn from rural or urban enumeration areas in Ghana, or from municipality registration and Ghanaian organizations in the three European sites. In Ghana, the participation rate was 76% at the rural and 74% at the urban recruitment sites. In Europe, the participation rates were 53% in Amsterdam, 75% in London and 68% in Berlin.
Data collection, measurements and definitions
Data collection was standardized using standard operation procedures across all sites. Information on demographics, socioeconomic position, migration-related factors, health status and health behaviour was obtained using questionnaires either self-administered or via interviews conducted by trained interviewers. Age showed a normal distribution, and was therefore presented as continuous variable. Education was used as a proxy for socioeconomic position, and was categorized into none or elementary schooling, lower secondary, higher secondary and tertiary education, or unknown level. First generation migrant was defined as being born in Ghana and migrated to Europe. Smoking status was categorized into current or former smoker, or never smoked. The World Health Organization STEPS questionnaire16 was used to derive physical activity in metabolic equivalent (MET, hours/week), which included physical activity at work, while commuting and in leisure time.17 Answers were subsequently classified based on the guidelines of The IPAQ group,18 into low, moderate or high level of physical activity.
Physical examinations were performed with validated devices. Weight and height were measured in light clothing without shoes using the SECA877 weighing scale and SECA217 portable stadiometer. WC was measured using measuring tape at the midpoint between the lower rib and the upper margin of the iliac crest. All anthropometric measurements were performed twice by the same examiner and the average of the two measurements was used for analysis. BP was measured three times after at least 5 min rest, in sitting position with an appropriate cuff, using Microlife WatchBP home, a semi-automated device. The mean of the last two measurements was used in the analysis.
Participants were instructed to fast from 10:00p.m. the night prior to the blood sample collection.
Fasting venous blood samples were collected by trained research assistants. Processing and transportation of the blood samples was standardized across all study sites.12 FPG, HDL-cholesterol and triglycerides were determined using the ABX Pentra 400 chemistry analyser (HORIBA ABX, Montpellier, France).
MetSyn was determined using the definition of International Diabetes Federation in collaboration with the National Heart, Lung, and Blood Institute, American Heart Association, World Heart Federation, International Atherosclerosis Society and International Association for the Study of Obesity.1 This definition identifies an individual with MetSyn if at least three of the following five criteria are present: elevated WC or abdominal obesity (men ≥94 cm, women ≥80 cm); elevated triglycerides (≥1.7 mmol/l and/or the use of lipid-lowering medication); reduced HDL-cholesterol (men <1.0 mmol/l, women <1.3 mmol/l, and/or the use of lipid-lowering medication); elevated BP (systolic ≥130 and/or diastolic ≥85 mmHg, and/or antihypertensive treatment); raised FPG (≥5.6 mmol/l and/or the use of blood glucose-lowering medication).
Statistical analysis
Participants aged 25–70 years were eligible for inclusion. 6385 Ghanaians agreed to participate in the study of which 5898 completed physical examination and provided blood samples for biochemical characterization. After exclusion of participants outside the age range, 5659 participants were included in the analysis.
All analyses were stratified by geographical location and sex. Age-standardized prevalence rates of MetSyn and its components were calculated using the age distribution of the total RODAM population as standard population. Prevalence ratios (PRs) with corresponding 95% confidence intervals (CI) were calculated using Poisson regression with model-based variance to compare the prevalence of MetSyn between the study sites.10,19 As the RODAM study conceptualized rural Ghana as the source from where Ghanaians migrated to urban Ghana and subsequently to Europe, we used rural Ghana as the reference category. In the Poisson regression analysis, adjustment was made for covariates in two models. Model 1 was adjusted for age and level of education; model 2 for age, level of education, physical activity and smoking status.10 The prevalence rates of MetSyn and its components were compared between the European sites, as this would potentially disclose differences between the populations of the three European study sites. Amsterdam was used as the reference population for the within-Europe comparison, as the population of this study location previously showed to have the highest prevalence of impaired fasting glucose.19 The analysis for the European sites was adjusted for the factors included in model 2 plus length of stay in Europe (in years).
Analyses were performed using IBM SPSS Statistics (SPSS Inc., Released 2016. SPSS for Windows, Version 24.0, Chicago, IL, USA). Age-standardized prevalence was calculated using R Statistics (R Core Team. 2017, R for Windows, version 3.4.1. R Foundation for Statistical Computing, Vienna, Austria).
Ethical approval and consent to participate
Ethical approval of the study was requested from the respective ethics committees in Ghana (School of Medical Sciences/Komfo Anokye Teaching Hospital Committee on Human Research, Publication & Ethical Review Board), the Netherlands (Institutional Review Board of the Academic Medical Center, University of Amsterdam), Germany (Ethics Committee of Charité-Universitätsmedizin Berlin) and the UK (London School of Hygiene and Tropical Medicine Research Ethics Committee) before data collection began in each country. Informed written consent was obtained from each participant prior to the enrolment in the study.
Results
Characteristics of study population
A total of 5659 participants were eligible for analysis, of which 62.3% were women. Table 1 shows detailed population characteristics of the included participants. The mean age of the sample was 46.2 years. Amsterdam-Ghanaian men, and London- and rural-Ghanaian women were older compared with participants in other sites. Ghanaians living in London were the most educated population. Ninety-seven percent of the European-Ghanaians were first generation migrants. Smoking prevalence was low in all locations, except among Berlin-Ghanaian men. Ghanaians living in London were least physically active.
Table 1.
Population characteristics by locality and sex
| Rural-Ghanaians | Urban-Ghanaians | Amsterdam-Ghanaians | Berlin-Ghanaians | London-Ghanaians | |
|---|---|---|---|---|---|
| Men, n (%) | 405 (19.0) | 415 (19.4) | 609 (28.5) | 297 (13.9) | 410 (19.2) |
| Age, years (CI) | 46.2 (45.0–47.5) | 46.5 (45.4–47.7) | 48.4 (47.7–49.2) | 45.8 (44.5–47.1) | 46.1 (45.0–47.1) |
| Education level, % (CI) | |||||
| None or elementary | 39.0 (34.4–43.8) | 22.2 (18.4–26.3) | 20.5 (17.5–23.9) | 6.1 (3.8–9.2) | 3.9 (2.3–6.1) |
| Lower secondary | 36.0 (31.5–40.8) | 42.4 (37.7–47.2) | 40.6 (36.7–44.5) | 47.8 (42.2–53.5) | 24.9 (20.9–29.2) |
| Higher secondary | 13.3 (10.3–16.9) | 20.5 (16.8–24.6) | 25.1 (21.8–28.7) | 28.3 (23.4–33.6) | 16.8 (13.4–20.7) |
| Tertiary | 5.7 (3.7–8.3) | 9.2 (6.7–12.2) | 8.2 (6.2–10.6) | 17.5 (13.5–22.1) | 41.0 (36.3–45.8) |
| Unknown | 5.9 (3.9–8.5) | 5.8 (3.8–8.3) | 5.6 (4.0–7.6) | 0.3 (0.0–1.6) | 13.4 (10.4–17.0) |
| Duration of stay in Europe, years (CI) | n/a | n/a | 18.7 (18.0–19.4) | 16.8 (15.5–18.2) | 15.1 (14.1–16.1) |
| First generation migrant, % (CI) | n/a | n/a | 98.6 (97.3–99.3) | 99.3 (97.9–99.9) | 98.6 (97.0–99.5) |
| Current smoking, yes, % (CI) | 5.8 (3.8–8.5) | 3.3 (1.9–5.4) | 8.1 (6.1–10.6) | 14.8 (11.1–19.2) | 1.3 (0.5–2.9) |
| Physical activity, % (CI) | |||||
| Low level | 10.8 (7.9–14.2) | 22.4 (18.5–26.7) | 20.6 (16.8–24.8) | 24.2 (19.6–29.3) | 41.4 (36.5–46.5) |
| Moderate level | 16.5 (13.1–20.5) | 18.6 (15.0–22.6) | 14.5 (11.3–18.2) | 20.9 (16.5–25.8) | 18.3 (14.6–22.4) |
| High level | 71.9 (67.3–76.3) | 57.5 (52.6–62.3) | 59.3 (54.4–64.1) | 52.2 (46.5–57.8) | 31.2 (26.6–36.0) |
| Women, n (%) | 638 (18.1) | 1034 (29.3) | 931 (26.4) | 250 (7.1) | 670 (19.0) |
| Age, years (CI) | 46.7 (45.7–47.6) | 44.7 (44.0–45.4) | 45.6 (45.0–46.1) | 44.7 (43.5–45.9) | 47.7 (46.9–48.5) |
| Education level, % (CI) | |||||
| None or elementary | 62.2 (58.4–65.9) | 50.5 (47.4–53.5) | 40.9 (37.7–44.0) | 11.6 (8.1–16.0) | 10.0 (7.9–12.4) |
| Lower secondary | 25.9 (22.6–29.4) | 35.9 (33.0–38.8) | 30.6 (27.7–33.7) | 54.0 (47.8–60.1) | 29.9 (26.5–33.4) |
| Higher secondary | 3.0 (1.9–4.5) | 8.5 (6.9–10.3) | 17.8 (15.5–20.4) | 24.8 (19.8–30.4) | 24.2 (21.1–27.5) |
| Tertiary | 1.9 (1.0–3.2) | 2.7 (1.8–3.8) | 3.8 (2.7–5.1) | 7.6 (4.8–11.4) | 22.1 (19.1–25.3) |
| Unknown | 7.1 (5.3–9.2) | 2.4 (1.6–3.5) | 6.9 (5.4–8.6) | 2.0 (0.8–4.3) | 13.9 (11.4–16.7) |
| Duration of stay in Europe, years (CI) | n/a | n/a | 17.7 (17.2–18.2) | 16.9 (15.7–18.2) | 17.4 (16.5–18.3) |
| First generation migrant, % (CI) | n/a | n/a | 99.5 (98.9–99.8) | 99.6 (98.1–100.0) | 96.9 (95.3–98.1) |
| Current smoking, yes, % (CI) | 0 (0.0) | 0.1 (0.0–0.5) | 2.1 (1.3–3.2) | 3.3 (1.5–6.0) | 0.2 (0.0–0.8) |
| Physical activity, % (CI) | |||||
| Low level | 23.2 (20.0–26.8) | 40.4 (37.4–43.4) | 16.5 (13.8–19.5) | 31.3 (25.8–37.3) | 41.6 (37.7–45.5) |
| Moderate level | 23.1 (19.8–26.6) | 15.7 (13.6–18.1) | 21.8 (18.8–25.1) | 18.3 (13.8–23.5) | 23.8 (20.6–27.3) |
| High level | 53.5 (49.5–57.5) | 43.2 (40.2–46.3) | 55.8 (52.0–59.6) | 45.5 (39.4–51.8) | 24.8 (21.5–28.3) |
Notes: Values are means or percentages with corresponding 95% confidence intervals. n, number; n/a, not available; CI, confidence interval.
Prevalence of MetSyn
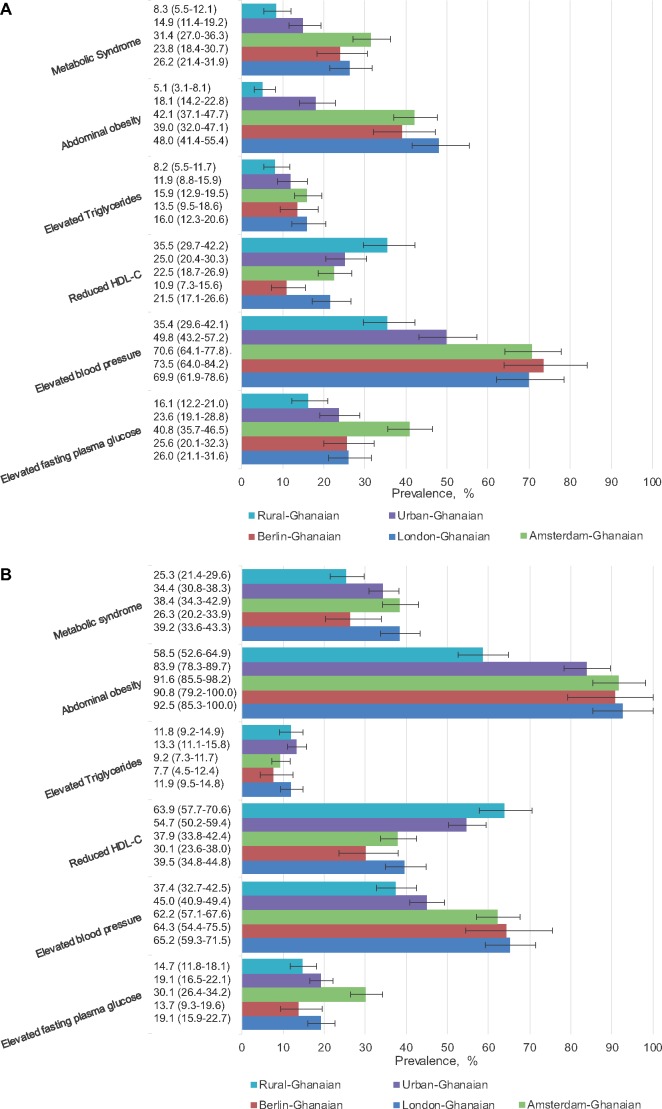
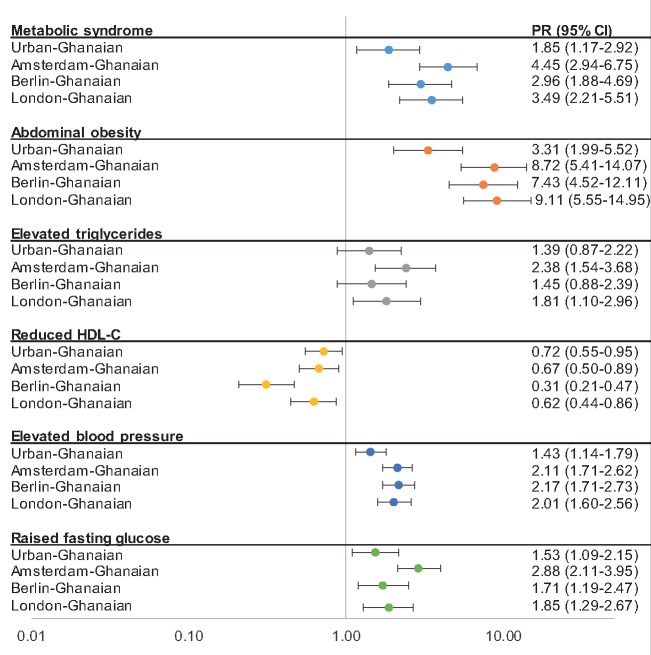
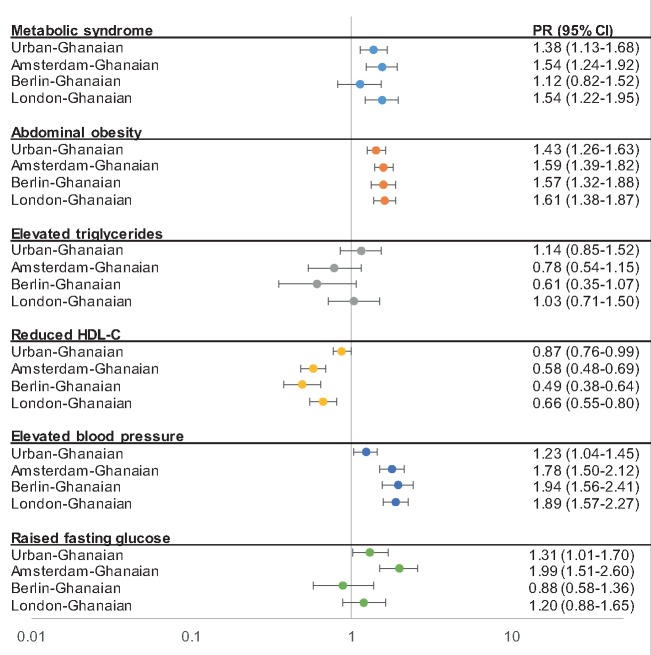
In men, the age-standardized MetSyn prevalence ranged from 8% (95% CI, 5.5–12.1) among rural-Ghanaians to 31% (27.0–36.3) among Amsterdam-Ghanaians, with a positive gradient in prevalence through urban Ghana to European sites (figure 1A). This positive gradient was also observed between rural- and urban-Ghanaian women [rural Ghana 25% (21.4–29.6), urban Ghana 34% (30.8–38.3)], but not between urban-Ghanaian and European-Ghanaian women (figure 1B). The adjusted PR of MetSyn was almost two times higher for urban-Ghanaian men compared with rural-Ghanaian men, and increased to a more than four times higher PR in Amsterdam-Ghanaian men (figure 2). Among women, PRs did not vary greatly between the sites (figure 3), except for Berlin-Ghanaian women, who had a lower PR compared with the urban-Ghanaian and the other European sites. Comparison between the European sites, with additional adjustment for length of stay in Europe, strengthened the trend towards a lower MetSyn PR in Berlin- compared with Amsterdam-Ghanaians (Supplementary figure S1).
Figure 1.
Age-standardized prevalence of metabolic syndrome, abdominal obesity, elevated triglycerides, reduced high-density lipoprotein cholesterol (HDL-C), elevated blood pressure and elevated fasting plasma glucose, by site in men (A) and women (B). Error bars are 95% confidence intervals
Figure 2.
Prevalence ratios (PRs) for metabolic syndrome and its components for men by site, compared with rural Ghana (reference population, PR = 1.00). Error bars are 95% confidence intervals. Model adjusted for age, level of education, physical activity and smoking status
Figure 3.
Prevalence ratios for metabolic syndrome and its components for women by site, compared with rural Ghana (reference population, PR = 1.00). Error bars are 95% confidence intervals. Model adjusted for age, level of education, physical activity and smoking status
Prevalence of MetSyn components
As the PRs for the MetSyn components did not vary between model 1 and model 2, only the results for model 2 are presented.
Abdominal obesity
In men, the age-standardized prevalence of abdominal obesity showed a positive rural–urban-Europe gradient (figure 1A). In women, abdominal obesity was highly prevalent at all sites, and showed a prevalence gradient between rural and urban Ghana, but not between urban Ghana and Europe (figure 1B). This was reflected in the PRs, which showed a positive rural–urban-Europe gradient in men (figure 2), but no difference between urban Ghana and Europe in women, compared with rural Ghana (figure 3). Moreover, the PRs did not differ between the European sites (Supplementary figure S1).
Elevated triglycerides
In men, the age-adjusted prevalence of elevated triglycerides did not vary between the locations, but a trend towards a higher prevalence in urban- and European-Ghanaians could be observed (figure 1A). In women, the prevalence tended to be higher in rural- and urban-Ghanaian women than in European-Ghanaian women (figure 1B).
Compared with rural-Ghanaian men, the adjusted PRs were twice as high in Amsterdam- and London-Ghanaian men, and comparable with urban- and Berlin-Ghanaian men. This was also reflected in the comparison of PRs between the European sites, which showed a lower PR for elevated triglycerides in Berlin- compared with Amsterdam-Ghanaian men (Supplementary Figure S1). In women, the PRs for elevated triglycerides were similar between study sites, with a trend towards lower PRs in Amsterdam and Berlin compared with rural Ghana (figure 3).
Reduced HDL-C
The age-adjusted prevalence of reduced HDL-C showed a negative gradient from rural Ghana, through urban Ghana to Europe, in both sexes, with the lowest prevalence of reduced HDL-C in Berlin-Ghanaians (figure 1A and B ). The adjusted PRs followed a similar pattern, with especially low PRs in Berlin- compared with rural-Ghanaian men and women (figures 2 and 3). Within the European sites, Berlin-Ghanaian men had a lower PR for reduced HDL-C compared with Amsterdam-Ghanaian men (Supplementary figure S1).
Elevated blood pressure
An elevated BP was more prevalent in Ghanaian migrants than participants residing in Ghana (figure 1A and B). The adjusted PRs showed a positive rural-urban-Europe gradient, with the PR being twice as high in the European sites compared with rural Ghana (figures 2 and 3). There were no differences in PRs between the European sites.
Raised fasting plasma glucose
In men, the age-adjusted prevalence and adjusted PRs of raised FPG showed a positive rural-to-urban gradient, with resembling prevalence between urban Ghana and Europe. In women, the prevalence rates were comparable between urban Ghana and London, and the adjusted PRs did not differ between rural Ghana, Berlin and London. In Europe, Amsterdam-Ghanaians had the highest prevalence of raised FPG (figure 1A and B), which was reflected in the PRs (Supplementary figure S1).
Discussion
Key findings
The MetSyn prevalence among Ghanaians differed by locality. In men, the MetSyn prevalence showed a positive gradient from rural Ghana, through urban Ghana to Europe, with a higher MetSyn prevalence in Amsterdam-Ghanaian men. In women, there was a positive gradient in MetSyn prevalence between rural- and urban-Ghanaian women. However, this prevalence did not differ between urban-Ghanaian women compared with their European-Ghanaian counterparts.
The prevalence rates of the individual MetSyn components—except for reduced HDL-C—were higher in urban Ghana compared with rural Ghana, and even higher in Europe. A decreased HDL-C level was more prevalent among rural-Ghanaians compared with their urban and European counterparts. There was a high prevalence of raised FPG in Amsterdam-Ghanaian men and women, which substantially accounted for the lower MetSyn prevalence in Berlin-Ghanaians compared with Amsterdam-Ghanaians.
Discussion of the key findings
The MetSyn prevalence showed a positive rural-to-urban gradient in both sexes, which seems to suggest that exposure to an urban environment, potentiates the development of metabolic risk factors.20 This is in line with findings from other SSA studies.21–23 Moreover, MetSyn occurred at nearly the same rate among urban-Ghanaian women compared with their European-Ghanaian counterparts, suggesting that similar underlying processes might be at play in urban areas in Ghana as in migrant populations in Europe.
The MetSyn prevalence is higher in Ghanaian migrant women than in women from the respective European host populations, whereas MetSyn is less prevalent in Ghanaian men compared with European men.11,24,25 These results are in line with findings from the USA, showing men from ethnic minorities being less affected by MetSyn, whereas women from ethnic minorities being more affected by MetSyn compared with the European descent population.26 The differences in MetSyn prevalence rates between the migrant and European host population is mostly attributable to the high prevalence of abdominal obesity in Ghanaian women.10,11,21,27 Genetic predisposition, differential exposures in early life and gene-environmental interactions might contribute to this disease susceptibility.12 Moreover, sociocultural perceptions regarding ideal body size may also contribute to the high abdominal obesity rate in women.28
The prevalence of raised FPG was remarkably higher among Amsterdam-Ghanaian migrants compared with Berlin- and London-Ghanaians and seems to be the major driving factor behind the differences in MetSyn prevalence between these European sites. These findings are in line with a previous study, showing a raised FPG prevalence of 35 and 14% in non-diabetic Amsterdam-African Caribbean’s and London-African Caribbean’s respectively, despite a higher prevalence of central obesity in the latter.10 The reason for the high susceptibility of Amsterdam-Ghanaian migrants for having raised FPG is not clear, but might be due to differences in contextual factors, such as differences in access to health care and preventive services,29 psychosocial factors30 or dietary habits. Moreover, these contextual factors could initiate epigenetic modifications, thereby shaping the glucose metabolism differently among similar populations living in different countries. Therefore, further research is required to clarify these intercountry variations.
Another interesting finding of our study is the high prevalence of reduced HDL-C in rural-Ghanaian residents, which showed to be significantly higher in this group compared with the prevalence in urban-Ghanaian and European-Ghanaian residents. These findings are in line with previous reports from Ghana and other SSA countries, demonstrating high prevalence rates of reduced HDL-C in both rural and urban populations, with higher prevalence rates in women than in men.7,23,31 These low levels of HDL-C in rural Ghana might be due to specific dietary habits, which appear to differ from the other geographical locations as previous RODAM results showed.32 As HDL-C has a key role in the transport of excessive cholesterol towards the liver for excretion, and has anti-thrombotic and anti-inflammatory properties, it fulfils an important cardioprotective role.33 Therefore, the high prevalence of reduced HDL-C in the Ghanaian population points to a potential important CVD risk factor and needs further research.
Strengths and limitations
This study is the first to look at MetSyn in a homogenous SSA migrant population in comparison to the population in their home country in SSA. Previous studies used migration surrogates (e.g. comparing native Africans with African-Americans) to study the effect of migration on CVD risk factors. However, people from the African diaspora originate from heterogeneous ancestry and show high levels of genetic diversity,34 which hampers the comparison with populations residing in SSA. Moreover, none of these migration studies focussed on MetSyn. The use of highly standardized operating procedures for data collection across all study sites made it possible to compare the different geographical locations. However, as the RODAM study has a cross-sectional study design, the possibilities to study the causality between MetSyn and the determinants tested in the statistical models are limited. In addition, as recruitment strategies had to be tailored to the different study locations, a certain selection bias might have occurred. However, as the non-response analysis revealed similar patterns between all sites, we consider it unlikely that the differences in prevalence rates between the sites are biased by the variations in sampling strategy. As we performed full case analysis, participants with missing data on education, smoking or physical activity were excluded from the Poisson regression analysis. However, the participants excluded from analysis did not differ from those included, in terms of age or sex. In addition, those with missing data did not differ from those with complete data with regards to prevalence of MetSyn or its components. Therefore, we do not expect missing data on these covariates to influence our results in a significant way. Lastly, different methods were used to administer the questionnaire, which could introduce some level of bias as varied methods could have influenced the participants’ answers differently. However, only small percentage of Ghanaian migrants in Europe (12%) self-administered the questionnaire. In addition, as research assistants were trained to conduct the interviews in a standardized way, we consider this limitation to be a minor source of bias.
Conclusion
Our findings show a positive gradient of the MetSyn prevalence from rural Ghana, through urban Ghana to Europe but only in men. The prevalence rates of the individual MetSyn components (abdominal obesity, elevated triglyceride, elevated BP and raised FPG) generally show positive gradients whereas reduced HDL-C shows a negative gradient from rural Ghana, through urban Ghana to Europe. In addition, the prevalence of MetSyn and its components vary among Ghanaian migrants living in different European countries. These distinctive geographical differences between the groups show the potential importance of contextual factors on cardiometabolic risk and suggest the need to unravel the underlying mechanisms to guide prevention management efforts in SSA populations.
Supplementary Material
Acknowledgements
The authors are very grateful to the advisory board members for their valuable support in shaping the methods, to the research assistants, interviewers and other staff of the five research locations who have taken part in gathering the data and, most of all, to the Ghanaian volunteers participating in this project. We gratefully acknowledge J. van Straalen from the Department of Clinical Chemistry, Academic Medical Centre (Amsterdam, The Netherlands) for his valuable support with standardization of the laboratory procedures, and the Academic Medical Centre (AMC) Biobank for support in biobank management and storage of collected samples.
Funding
This work was supported by the European Commission under the Framework Programme (Grant Number: 278901). Liam Smeeth’s contribution was supported by the Wellcome Trust, grant number WT082178. J.S. was supported by the DZHK (German Centre for Cardiovascular Research) and the Berlin Institute of Health (BIH).
Conflicts of interest: None declared.
Key points
Metabolic syndrome (MetSyn) is an important risk factor for cardiovascular diseases and type 2 diabetes. It is unknown whether the rate of metabolic syndrome differs between rural and urban settings in Africa and upon migration to Europe.
This study shows a positive rural-to-urban gradient in MetSyn prevalence in both men and women, and a higher prevalence in European- than in urban-Ghanaian men, but no differences in prevalence between urban-Ghanaian and European-Ghanaian women.
The prevalence rates of the individual MetSyn components—except for reduced HDL-C—are higher in urban Ghana compared with rural Ghana, and even higher in Europe; reduced HDL-C is more prevalent in rural Ghana than in the other geographical locations.
Difference in MetSyn prevalence between the European sites is mostly attributable to the high prevalence of raised fasting plasma glucose in Amsterdam-Ghanaians.
To assist preventive efforts, further research is needed to understand mechanisms driving the geographical differences in MetSyn prevalence.
References
- 1. Alberti K, Eckel RRH, Grundy SSM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation task force on epidemiology and prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009;120:1640–5. [DOI] [PubMed] [Google Scholar]
- 2. Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care 2005;28:1769–78. [DOI] [PubMed] [Google Scholar]
- 3. Ford ESE, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008;31:1898–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol 2010;56:1113–32. [DOI] [PubMed] [Google Scholar]
- 5. Murphy GAV, Asiki G, Young EH, et al. Cardiometabolic risk in a rural Ugandan population. Diabetes Care 2013;36:e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ofori-Asenso R, Agyeman AA, Laar A. Metabolic syndrome in apparently “healthy” Ghanaian adults: a systematic review and meta-analysis. Int J Chronic Dis 2017;2017:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kaduka LU, Kombe Y, Kenya E, et al. Prevalence of metabolic syndrome among an urban population in Kenya. Diabetes Care 2012;35:887–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mbanya JCN, Motala AA, Sobngwi E, et al. Diabetes in sub-Saharan Africa. Lancet 2010;375:2254–66. [DOI] [PubMed] [Google Scholar]
- 9. Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United States, 2003–2006. Natl Health Stat Rep 2009;1–7. [PubMed] [Google Scholar]
- 10. Agyemang C, Kunst AEA, Bhopal R, et al. A cross-national comparative study of metabolic syndrome among non-diabetic Dutch and English ethnic groups. Eur J Public Health 2013;23:447–52. [DOI] [PubMed] [Google Scholar]
- 11. Agyemang C, van Valkengoed IG, van den Born BJ, et al. Heterogeneity in sex differences in the metabolic syndrome in Dutch white, Surinamese African and South Asian populations. Diabet Med 2012;29:1159–64. [DOI] [PubMed] [Google Scholar]
- 12. Agyemang C, Beune E, Meeks K, et al. Rationale and cross-sectional study design of the Research on Obesity and type 2 Diabetes among African Migrants: the RODAM study. BMJ Open 2015;4:e004877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centraal Bureau voor de Statistiek. Migratie; land van herkomst/vestiging, geboorteland en geslacht [Internet], 2018. Available at: https://opendata.cbs.nl/#/CBS/nl/dataset/60032/table? ts=1545235009446 (2 January 2019, date last accessed).
- 14.German Technical Cooperation (GTZ). Die ghanaische Diaspora in Deutschland—Ihr Beitrag zur Entwicklung Ghanas, Eschborn, 2009.
- 15.Office for National Statistics. Population of the UK by country of birth and nationality [Internet], 2018. Available at: https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/internationalmigration/datasets/populationoftheunitedkingdombycountryofbirthandnationality (2 January 2019, date last accessed).
- 16. Brathwaite R, Addo J, Kunst AE, et al. Smoking prevalence differs by location of residence among Ghanaians in Africa and Europe: the RODAM study. PLoS One 2017;12:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ainsworth BE, Haskell WL, Herrmann SD, et al. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011;43:1575–81. [DOI] [PubMed] [Google Scholar]
- 18.Guidelines for data processing and analysis of the International Physical Activity Questionnaire (IPAQ)—short and long forms [Internet], 2005:1–15. Available at: https://sites.google.com/site/theipaq/scoring-protocol (2 October 2017, date last accessed).
- 19. Agyemang C, Meeks K, Beune E, et al. Obesity and type 2 diabetes in sub-Saharan Africans—is the burden in today’s Africa similar to African migrants in Europe? The RODAM study. BMC Med 2016;14:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Abubakari AR, Lauder W, Jones MC, et al. Prevalence and time trends in diabetes and physical inactivity among adult West African populations: the epidemic has arrived. Public Health 2009;123:602–14. [DOI] [PubMed] [Google Scholar]
- 21. Assah FK, Ekelund U, Brage S, et al. Urbanization, physical activity, and metabolic health in sub-Saharan Africa. Diabetes Care 2011;34:491–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adediran O, Akintunde AA, Edo AE, et al. Impact of urbanization and gender on frequency of metabolic syndrome among native Abuja settlers in Nigeria. J Cardiovasc Dis Res 2012;3:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kodaman N, Aldrich MC, Sobota R, et al. Cardiovascular disease risk factors in Ghana during the rural-to-urban transition: a cross-sectional study. PLoS One 2016;11:e0162753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Williams ED, Steptoe A, Chambers JC, Kooner JS. Ethnic and gender differences in the relationship between hostility and metabolic and autonomic risk factors for coronary heart disease. Psychosom Med 2011;73:53–8. [DOI] [PubMed] [Google Scholar]
- 25. Ford ES, Schulze MB, Pischon T, et al. Metabolic syndrome and risk of incident diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study. Cardiovasc Diabetol 2008;7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, National Health and Nutrition Examination Survey, 1988–2012. Prev Chronic Dis 2017;14:160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sabir AA, Jimoh A, Iwuala SO, et al. Metabolic syndrome in urban city of North-Western Nigeria: prevalence and determinants. Pan Afr Med J 2016; 23:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tuoyire DA, Kumi-Kyereme A, Doku DT, Amo-Adjei J. Perceived ideal body size of Ghanaian women: “Not too skinny, but not too fat.” Women Health 2018;58: 583–97. [DOI] [PubMed] [Google Scholar]
- 29. Agyemang C, de-Graft Aikins A, Bhopal R. Ethnicity and cardiovascular health research: pushing the boundaries by including comparison populations in the countries of origin. Ethn Health 2012;17:579–96. [DOI] [PubMed] [Google Scholar]
- 30. Ikram UZ, Snijder MB, Agyemang C, et al. Perceived ethnic discrimination and the metabolic syndrome in ethnic minority groups: the healthy life in an urban setting study. Psychosom Med 2017;79:101–11. [DOI] [PubMed] [Google Scholar]
- 31. van Zyl S, van der Merwe LJ, Walsh CM, et al. Risk-factor profiles for chronic diseases of lifestyle and metabolic syndrome in an urban and rural setting in South Africa. Afr J Prim Health Care Fam Med 2012;4:1–10. [Google Scholar]
- 32. Galbete C, Nicolaou M, Meeks KA, et al. Food consumption, nutrient intake, and dietary patterns in Ghanaian migrants in Europe and their compatriots in Ghana. Food Nutr Res 2017;61:1341809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Remaley AT, Amar M, Sviridov D. HDL-replacement therapy: mechanism of action, types of agents and potential clinical indications. Expert Rev Cardiovasc Ther 2008;6:1203–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tishkoff SA, Reed FA, Friedlaender FR, et al. The genetic structure and history of Africans and African Americans. Science 2009;324:1035–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.