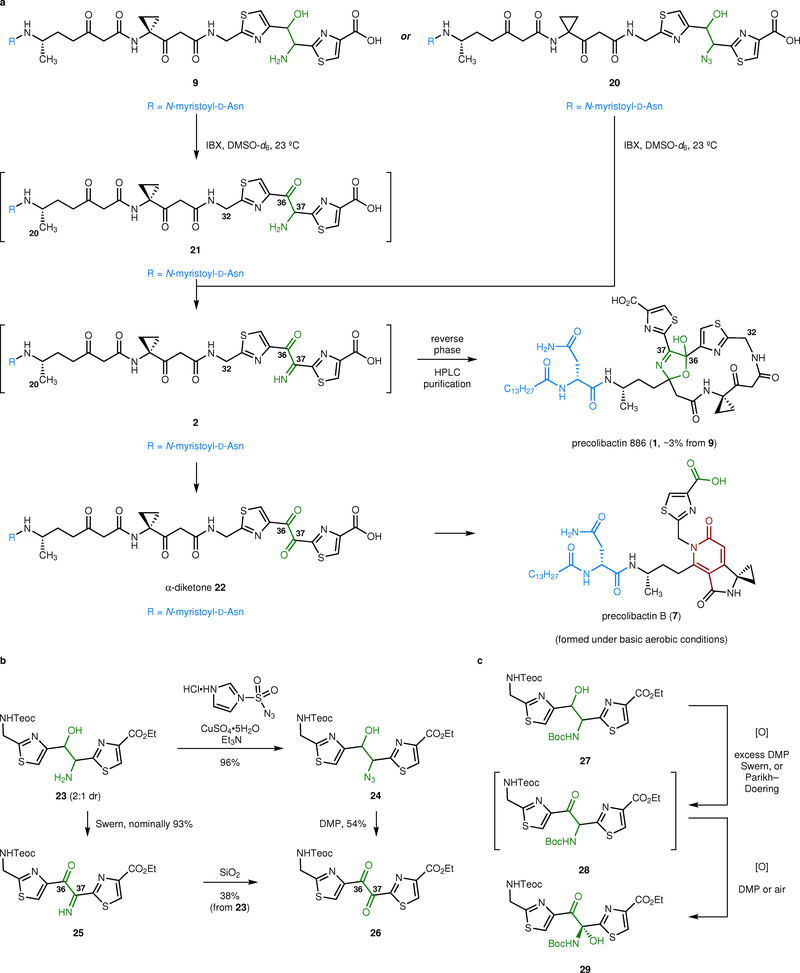
Fig. 3.
Synthesis of precolibactin 886 (1) and reactivity of the α-ketoimine and α-dicarbonyl residues. a, Two-fold oxidation of the 1,2-aminoalcohol 9 generates the α-ketoimine 2. This intermediate is unstable toward hydrolysis to the α-diketone 22. We were unable to effect the cyclization of 2 under a broad range of acidic, basic, or thermal conditions. However, we found that the α-ketoimine 2 converted to precolibactin 886 (1) upon HPLC purification. Oxidation of the azidoalcohol 20 with excess IBX also formed the α-ketoimine 2, presumably by loss of dinitrogen from an α-ketoazide intermediate (not shown). b, Synthesis of the α-ketoimine 25 and the α-dicarbonyl 26. The α-ketoimine 25 partially hydrolyzed to the α-dicarbonyl 26 upon exposure to water during work-up. Alternatively, this intermediate could be deliberately transformed to 26 by exposure to silica gel. c, Oxidation of the N-(tert-butoxycarbonyl)-1,2-amino alcohol 27. Oxidation with excess DMP generated the N-(tert-butoxycarbonyl) aminal 29. Alternatively, oxidation with 0.9 equiv of DMP formed the α-aminoketone 28. The α-aminoketone 28 transformed spontaneously to the hemiaminal 29 on standing.