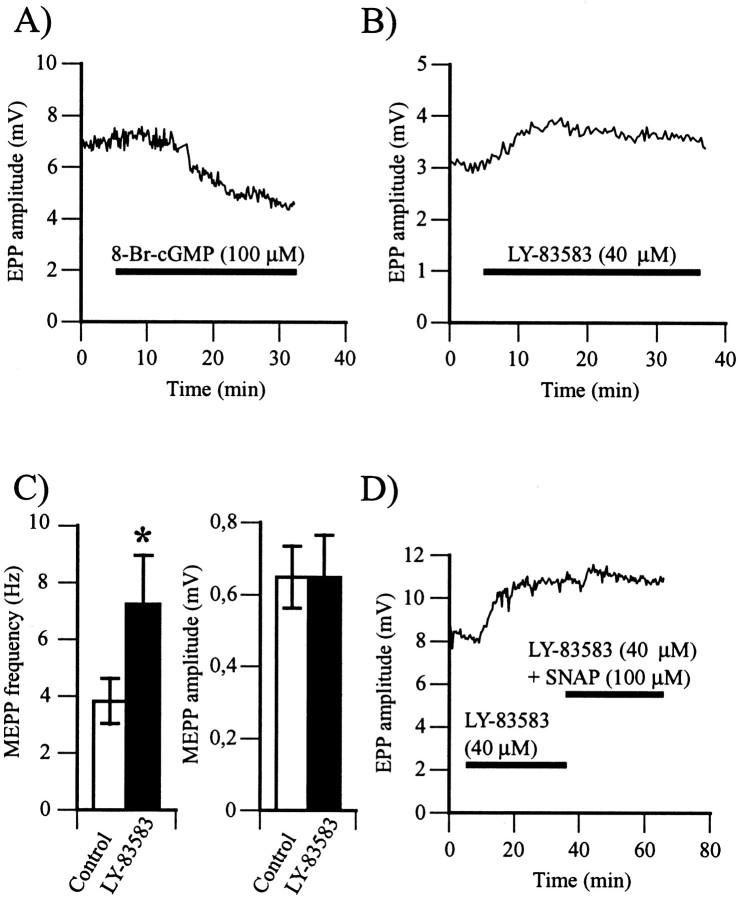
Fig. 4.
NO activates the soluble guanylate cyclase–cGMP-dependent pathway. A, Changes in EPP amplitude before and during bath application of 8-Br-cGMP (100 μm), a cell-permeable cGMP analog. The horizontal bar represents the period of exposure to the cGMP analog. Similar results were obtained in six experiments. B, Changes in EPP amplitude before and during bath application of LY-83583 (40 μm), a soluble guanylate cyclase antagonist. Thehorizontal bar represents the period of exposure to LY-83583. Note the rise in EPP amplitude in the presence of the soluble guanylate cyclase antagonist. Similar results were obtained in 14 experiments. C, Left, Histogram of the mean ± SEM of MEPP frequency in control (open bar) and after a 30 min exposure to LY-83583 (filled bar) (*p < 0.05; Student's one-tail pairedt test). Right, Histogram of the mean ± SEM of MEPP amplitude in control (open bar) and after a 30 min exposure to LY-83583 (filled bar). In six experiments, LY-83583 raised MEPP frequency and had no effects on MEPP amplitude. D, Changes in EPP amplitude in control and during bath application of LY-83583 and of LY-83583 simultaneously with SNAP. The horizontal barsrepresent exposure to the different drugs. In six experiments, LY-83583 (40 μm) increased EPP amplitude, and subsequent application of SNAP (100 μm) had no effect.