Fig. 3.
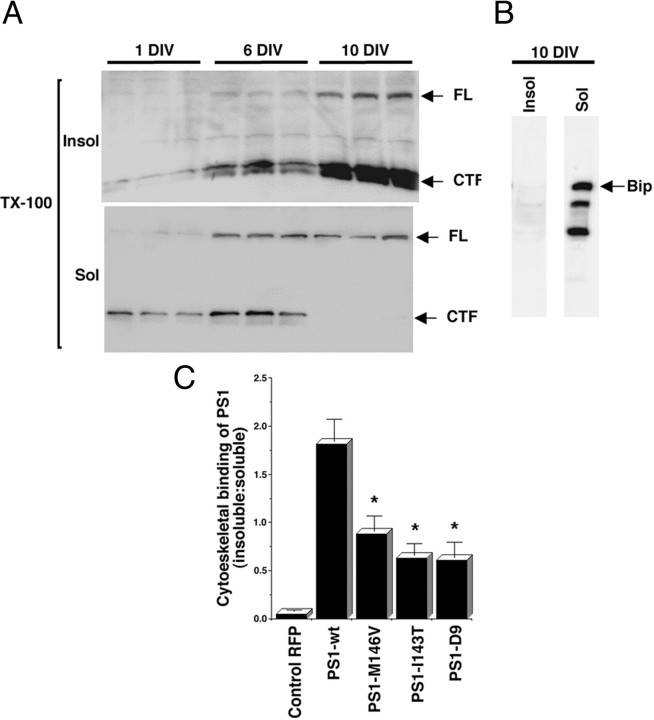
The cytoskeletal association of PS1 increases during neuronal development, and PS1 mutations reduce cytoskeletal binding. A, Western blot analysis of cytoskeletal preparations of 1, 6, and 10 DIV cultures. Shown are insoluble (Insol) and soluble (Sol) fractions prepared from triplicate cultures. All lanes were loaded with 10 μg of protein. The blots were developed with antibody AD3L that recognizes PS1 FL and CTF. Note the dramatic increase in the association of FL and CTF to the insoluble fraction during neuronal development. In contrast, the ER-resident protein Bip is solubilized completely by extraction with TX-100 (B).C, Cytoskeletal binding of RFP and PS1 expressed as the amount of protein in the insoluble-over-soluble fractions, determined by quantitative Western blot analysis with antibody AD3L to PS1 CTF and anti-RFP (see Materials and Methods). Cultures were transfected at 8 DIV and analyzed at 10 DIV. Similar results were obtained with antibodies PSN2 and 2025 recognizing PS1 NTF (data not shown). Note that PS1-wt is retained more efficiently in the cytoskeleton than PS1 mutations. Values are the mean ± SE; n = 6 independent experiments. *p < 0.001 relative to PS1-wt by Student's t test.