Fig. 1.
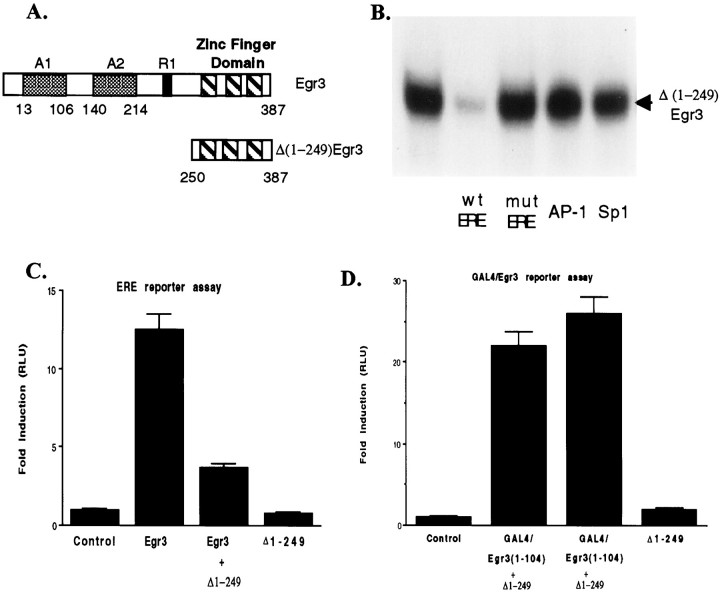
Characterization of the Egr inhibitor construct Δ(1-249)Egr3. A, Top, Thebar shows a schematic diagram of Egr3 that contains three zinc finger motifs near its C terminal that mediate its interaction with the ERE, two distinct activation domains, A1 and A2, and a modulatory domain, R1, that serves as a binding site for NAB1 and NAB2. Bottom, The bar illustrates the Egr inhibitor construct Δ(1-249)Egr3 designed to retain the DNA-binding domain without the upstream activation or modulatory domains.B, The autoradiogram illustrates that the Egr inhibitor construct forms a gel-shift complex with the ERE oligonucleotide probe. Formation of this complex is blocked by addition of unlabeled ERE [wild-type ERE (wt ERE)] but not a mutant ERE (mut ERE) in which a single base pair has been changed. (Wild-type and mutant ERE oligonucleotide sequences are provided in Materials and Methods.) Unlabeled ERE oligonucleotides were added at the same concentration as the ERE probe (∼0.5 nm). In addition, neither AP-1 nor Sp1 oligonucleotides inhibit binding of the Egr inhibitor construct to the ERE, when added at 500-fold higher concentration than the ERE probe. C, HEK293 cells were transfected with a luciferase reporter plasmid containing a tandem repeat of ERE sites in its promoter as well as with the other expression plasmids listed under eachcolumn of the bar graph [Egr3 plasmid, 0.5 μg/well; Δ(1-249)Egr3, 0.5 μg/well]. The ability of Egr3 to increase luciferase activity in extracts from these cells was blocked by the Egr inhibitor construct. As expected, the Egr inhibitor construct was unable to increase luciferase activity compared with that in control cells that only received the ERE luciferase reporter plasmid.D, HEK293 cells were transfected with a luciferase reporter plasmid containing a tandem repeat of GAL4 response elements in its promoter as well as with the other expression plasmids listedunder each column. GAL4/Egr3(1-104) refers to a chimeric protein generated by fusing the GAL4 DNA-binding domain with the A1 activation domain of Egr3. In contrast to its ability to block stimulation of reporter gene expression by full-length Egr3, the Egr inhibitor construct [Δ(1-249)Egr3; 0.5 μg/well] does not block the increase in luciferase activity driven by the GAL4/Egr3(1-104) construct (0.5 μg/well) acting on a GAL4 reporter plasmid. In C and D and the figures that follow, “Δ 1-249” refers to Δ(1-249)Egr3. Error bars shown in this and subsequent figures represent the SEM. Data shown inC and D are presented in relative luciferase units (RLU).