Fig. 5.
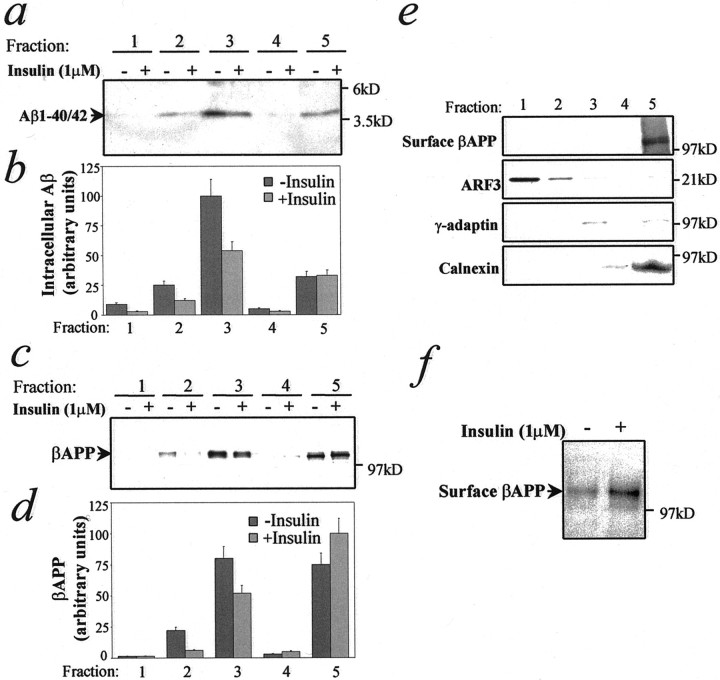
Insulin influences Aβ and βAPP trafficking in N2a cells. Cells were treated for 16 hr in the absence or presence of 1 μm insulin, homogenized, and fractionated on an equilibrium flotation sucrose gradient (see Materials and Methods).a, Representative autoradiographic analysis and quantitative analysis (b) of Aβ subcellular distribution after insulin treatment. c, Representative autoradiographic analysis and quantitative analysis (d) of intracellular βAPP subcellular distribution after insulin treatment. e, Markers for subcellular compartments. Proteins from each fraction were precipitated by trichloroacetic acid and analyzed by Western blot, using the antibodies anti-calnexin (ER), anti-γ-adaptin (TGN), or anti-ARF3 (post-TGN vesicles, cytosol). Surface βAPP (plasma membrane) was determined as described (see Materials and Methods). Fraction 1 = 0.25 m sucrose solution (loading, cytosol). Fractions 2–5 correspond, respectively, to interfaces between 0.25/0.8 m (post-TGN vesicles), 0.8/1.16m (Golgi/TGN), 1.16/1.3 m, and 1.3m/2 m (heavy membranes such as ER, plasma membranes) sucrose solutions. f, N2a cells were treated for 4 hr in the absence or presence of 1 μminsulin. Surface proteins were labeled with biotin. Biotinylated βAPP was analyzed by immunoprecipitation with 369 antibody and Western blot with HRP-conjugated streptavidin.