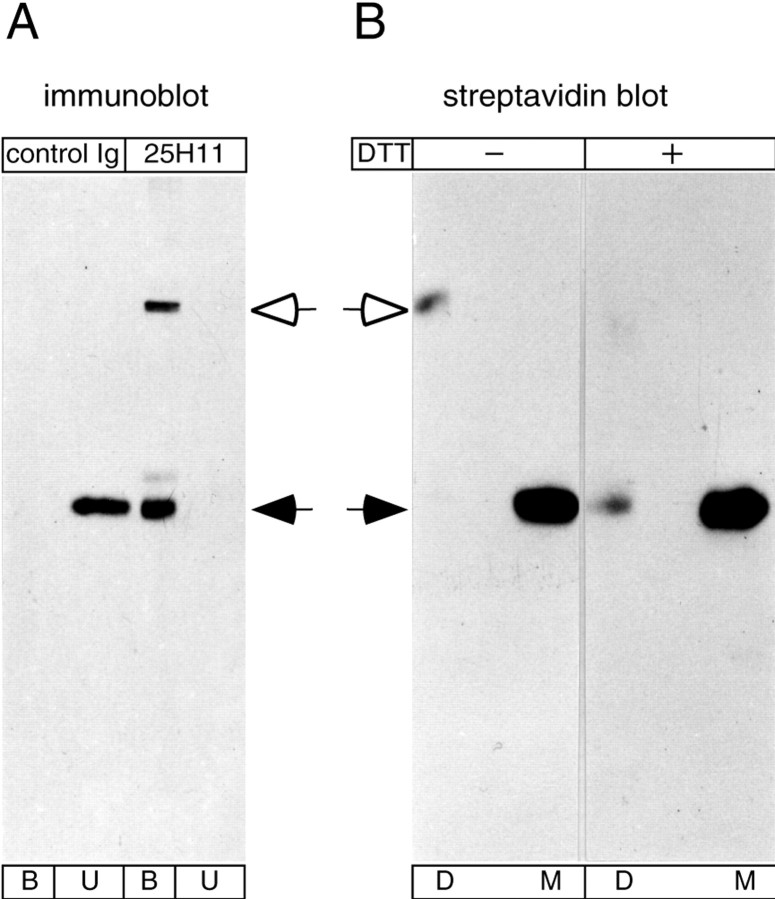
Fig. 4.
Disulfide-mediated homodimerization of the 25H11 antigen during immunoaffinity isolation. A, Aliquots of a Triton X-100 lysate obtained from 10 E13.5 mouse telencephali as described in Figure 11A were incubated with 25H11-Sepharose (25H11) or Sepharose conjugated with normal rat Igs (control Ig), followed by 25H11 immunoblot analysis of the unbound proteins (U) and the bound proteins eluted from the Sepharose (B).B, The 25H11 antigen was purified through the 25H11-Sepharose step as described in Figure 11A, except that the Triton X-100 lysate of only ≈55 E13.5 mouse telencephali was used. An aliquot of the eluate from the 25H11-Sepharose was biotinylated and subjected to SDS-PAGE under nonreducing conditions, and the positions of biotinylated proteins in the gel were determined by streptavidin blotting of thin stripes of the gel. Gel pieces containing either the dimer (D) or monomer (M) of the 25H11 antigen were treated without (−) or with (+) DTT followed by SDS-PAGE and streptavidin blotting. To facilitate comparison, the exposure shown for the reduced samples is longer than that shown for the nonreduced samples. A, B,Open arrows, dimer, andfilled arrows, monomer, of the 25H11 antigen.