Fig. 5.
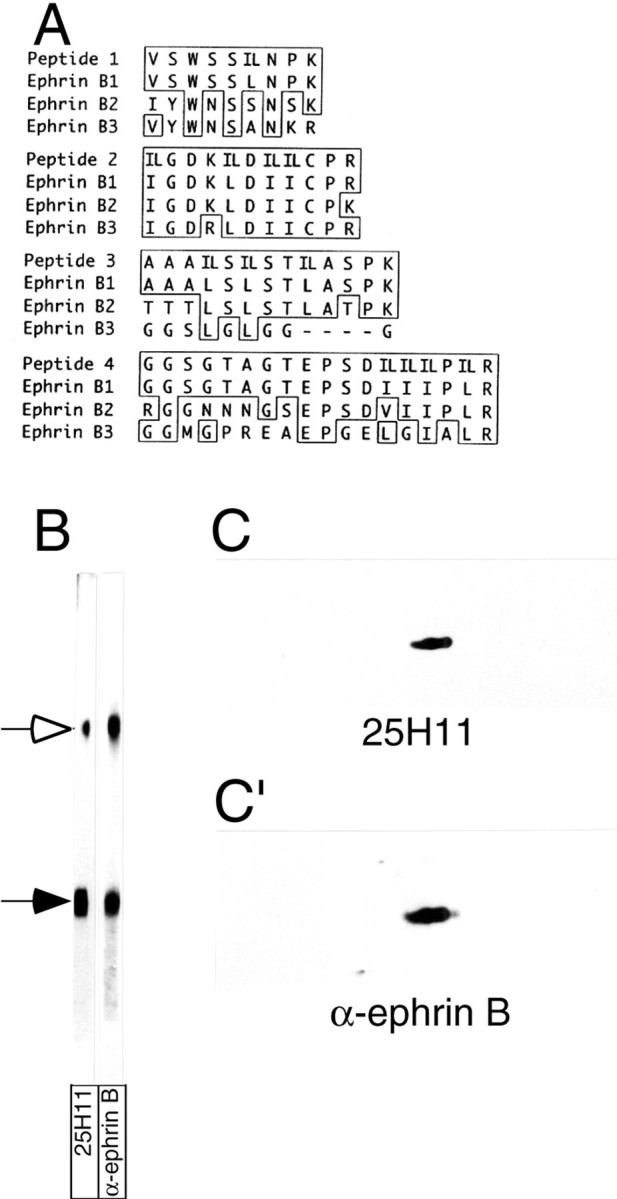
Identification of the 25H11 antigen as ephrin B1.A, The 25H11 antigen was purified as described in Figure3A, and digested with trypsin, and the sequence of four peptides was determined by nanoelectrospray mass spectrometry. The sequences are shown compared with the corresponding sequences of the members of the mouse ephrin B family; numbers refer to the sequences including the signal peptides. Identical amino acid residues are boxed; leucine (L) and isoleucine (I), which could not be distinguished by mass spectrometry, were equally considered in the comparison of peptides 1–4 with the ephrin B family members (IL). Peptides 1–4 were obtained from the 47 kDa monomer of the 25H11 antigen; peptides 3 and 4 were independently obtained from the dimer of the 25H11 antigen. Note that peptides 2–4 match to predicted internal tryptic peptides of ephrin B1, whereas peptide 1 corresponds to the C-terminal portion of the predicted N-terminal tryptic peptide of mature ephrin B1. B, The 25H11 antigen was purified through the 25H11-Sepharose step as described in Figure 3A, except that the Triton X-100 lysate of only ≈10 E13.5 mouse telencephali was used. An aliquot of the eluate from the 25H11-Sepharose was subjected to SDS-PAGE followed by transfer to nitrocellulose, which was cut into two stripes and immunolabeled using mAb 25H11 (25H11) or an antiserum against the ephrin Bfamily members (α-ephrin B). Open arrow, Dimer of the 25H11 antigen; filled arrow, monomer. Note that the immunoreactivity of the dimer band is less for mAb 25H11 as compared with α-ephrin B because of unequal cutting of the nitrocellulose. C, C′, pH11 carbonate-treated membranes prepared from four E13.5 mouse telencephali were analyzed by two-dimensional PAGE (acidic, right) followed by immunoblotting with the 25H11 antibody (C, ≈5 min time ECL exposure). The nitrocellulose was incubated in reducing condition to remove the antibody and reprobed with an antiserum against the ephrin B family members (C′, ≈1 min time ECL exposure).
