Fig. 2.
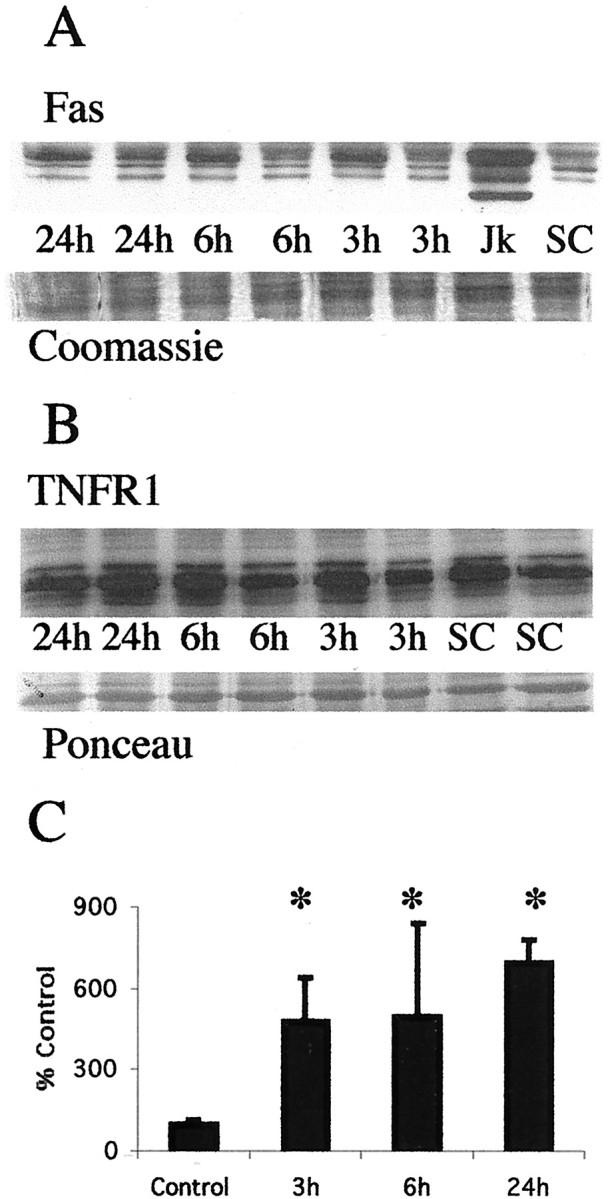
Fas death receptor protein levels increase in the thalamus after neonatal hypoxia–ischemia. A, Top, Immunoblot showing increased Fas death receptor protein in membrane fractions from thalamic homogenates obtained 3, 6, and 24 hr after neonatal hypoxia–ischemia compared with noninjured control samples. Jurkat cell lysates were used as a positive control, because they express high levels of Fas as detected at 45 kDa. The corresponding 45 kDa band in controls and injured thalamic samples was used for quantification. Bottom, The corresponding Coomassie-stained gel. B, Top, Immunoblot showing no change in TNFR1 death receptor protein in membrane fractions from thalamic homogenates obtained 3, 6, and 24 hr after neonatal hypoxia–ischemia compared with noninjured control samples.Bottom, The corresponding Ponceau-stained blot. ForA and B each lanerepresents a pooled sample of thalamus from three animals at the indicated time point (i.e., sham control and 3, 6, or 24 hr after hypoxia–ischemia). C, Graph representing changes in Fas death receptor protein levels in the thalamus over time after neonatal hypoxia–ischemia. Results are shown as the mean ± SD of four to five pooled samples per time point (*p < 0.05 compared with control). Jk, Jurkat cell lysates;SC, sham control.
