Abstract
Temperamentally anxious individuals can be identified in childhood and are at risk to develop anxiety and depressive disorders. In addition, these individuals tend to have extreme asymmetric right prefrontal brain activity. Although common and clinically important, little is known about the pathophysiology of anxious temperament. Regardless, indirect evidence from rodent studies and difficult to interpret primate studies is used to support the hypothesis that the amygdala plays a central role. In previous studies using rhesus monkeys, we characterized an anxious temperament endophenotype that is associated with excessive anxiety and fear-related responses and increased electrical activity in right frontal brain regions. To examine the role of the amygdala in mediating this endophenotype and other fearful responses, we prepared monkeys with selective fiber sparing ibotenic acid lesions of the amygdala. Unconditioned trait-like anxiety–fear responses remained intact in monkeys with >95% bilateral amygdala destruction. In addition, the lesions did not affect EEG frontal asymmetry. However, acute unconditioned fear responses, such as those elicited by exposure to a snake and to an unfamiliar threatening conspecific were blunted in monkeys with >70% lesions. These findings demonstrate that the primate amygdala is involved in mediating some acute unconditioned fear responses but challenge the notion that the amygdala is the key structure underlying the dispositional behavioral and physiological characteristics of anxious temperament.
Keywords: rhesus monkey, anxiety, fear, amygdala, temperament, EEG
The amygdala is a structure that is located in the medial temporal lobe and is composed of numerous subnuclei. The basolateral regions receive information from areas such as the cortex and thalamus, and the central nucleus sends efferents to projection sites that are important in mediating the behavioral, autonomic, and endocrine responses to stressors. Since the early studies of Kluver and Bucy (1939) and Weiskrantz (1956) the amygdala has been hypothesized to be a critical structure in mediating fear, anxiety, and other defensive behaviors. These studies, performed in rhesus monkeys with large lesions of the temporal lobe, reported dramatic effects such that monkeys with feral behavior became tame. These initial studies were a major impetus for numerous other investigators to explore amygdala functions in relation to emotion and behavior. Studies in rodents demonstrated that the amygdala is a key component of the neural circuitry underlying acute fear responses, as well as in the acquisition and expression of conditioned fear (Blanchard and Blanchard, 1972; Ledoux et al., 1988; Davis, 1992). However, the rodent data are difficult to interpret in relation to human behavior and psychopathology because there are important differences between rodents and primates in behavior, amygdala anatomy, and amygdala–prefrontal cortical circuitry (Amaral et al., 1992;Kalin, 1993). A small number of studies have been performed in nonhuman primates in an attempt to examine the role of the amygdala in mediating emotion. The results from these studies are difficult to interpret because the lesions were nonspecific, damaging overlying cortical and hippocampal regions. In addition to destroying cell bodies, these lesions also destroyed fibers coursing through the amygdala (Aggleton and Passingham, 1981; Kling and Brothers, 1992). Functional neuroimaging studies demonstrate that the human amygdala is activated during negative affective states such as sadness and anxiety (Davidson and Irwin, 1999). However, data from imaging studies are correlative and do not address mechanisms. A handful of patients with relatively selective lesions of the amygdala have been identified, and results from these patients are consistent with a role for the amygdala in processing negative emotions (Adolphs et al., 1994). In addition, studies suggest that the amygdala may be overactive in patients with depression and certain anxiety disorders (Drevets and Raichle, 1995;Davidson and Irwin, 1999). To develop a link between the large corpus of mechanistic data in rodents and the correlational findings from human imaging studies, we have been studying the role of the amygdala in mediating emotion and some of its physiological concomitants in rhesus monkeys.
As a prelude to investigating the neural circuitry mediating normal and pathological fear and anxiety responses in primates, we characterized the behavior and physiological endophenotype of anxious temperament in rhesus monkeys (Kalin and Shelton, 1989; Kalin, 1993). Chronically fearful or anxious monkeys have trait-like exaggerated defensive or fear-related responses and a pattern of brain activity that is associated with anxiety and dispositional negative effect in humans (Kalin et al., 1998; Davidson and Irwin, 1999). These monkeys exhibit extreme asymmetric right frontal brain electrical activity (Kalin et al., 1999) as well as increased basal levels of plasma cortisol (Kalin et al., 1998) and increased CSF corticotropin-releasing hormone concentrations (Kalin et al., 2000). We suggested that the behavioral responses of these monkeys, such as excessive freezing in the presence of a human, are similar to those described in temperamentally anxious children who are extremely shy and behaviorally inhibited in novel situations and when confronted by strangers (Kalin and Shelton, 1989). In children, extreme socially induced behavioral inhibition has been hypothesized to be mediated by increased amygdala activity (Kagan et al., 1988) and is associated with anxious temperament and with the later development of anxiety and depressive disorders (Biederman et al., 1993). In addition, marked social inhibition is characteristic of adults with anxiety disorders, such as social phobia. Studies in humans and monkeys demonstrate that extreme asymmetric right frontal electrical activity is associated with a dispositionally negative or anxious temperament (Davidson, 1995). To assess the role of the primate amygdala in mediating acute fearful responses, as well as the dispositional behavioral and physiological features of anxious temperament, we lesioned the amygdalas in 17 rhesus monkeys (Macaca mulatta) with ibotenic acid. The effects of the lesions on relevant behavioral and physiological parameters were assessed.
MATERIALS AND METHODS
Experimental subjects. Rhesus monkeys (M. mulatta) were used as experimental subjects. The animals were housed at the Harlow Center for Biological Psychology and at the Wisconsin Regional Primate Research Center. Animal housing and experimental procedures were in accordance with institutional guidelines. Seventeen animals (15 males and 2 females) underwent lesioning procedures at an average age of 27.9 months. Ten unoperated controls (nine males and one female) were used for comparison and were on average 22.5 months of age at the beginning of the study.
Amygdala lesions. The method for producing amygdala lesions was adapted from previously described methods (Amaral and Price, 1983;Murray and Mishkin, 1998). Because of the variability in rhesus monkey brain size, individual magnetic resonance images (MRIs) are necessary to define the boundaries of the amygdala. In addition, external landmarks were placed on the skull surface above the amygdala to be used as reference points in determining the stereotactic coordinates for lesioning in relation to the MRI. To further refine the stereotactic coordinates in the dorsoventral plane, an electrophysiological recording of cells at the top of the amygdala and at the bottom of the brain was performed.
External landmarks were established by stereotactically implanting two 3 mm glass beads filled with a 3% solution of copper sulfate (hyperintense in T1-weighted MRI images) into shallow indentations in the skull. The beads were placed at the mid-anteroposterior level of the amygdala (11 mm lateral to the midline and 9 and 15 mm anterior to the interaural line) and were cemented into place with dental acrylic.
Approximately 1 week later, an MRI was obtained to localize the beads implanted on the skull surface in relation to the amygdala. After administration of ketamine (15 mg/kg), the monkey was placed in a plastic replica of a Kopf stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) positioned in a small head coil. Using a 1.5 tesla GE Signa scanner, the brain was imaged in the coronal plane to verify symmetric alignment to the stereotaxic apparatus and the scanner. The animal was then scanned in the sagittal plane to establish the position of the bead in relation to each coronal slice. To exactly specify the location of the beads for definition of the lesion coordinates, coronal scans were obtained using a three-dimensional fast-spoiled gradient pulse sequence (3D/FSPGR/20) with a repetition rate of 11.5, a fractional echo time of 2.2/F, one echo, a receiver bandwidth of 15.6 kHz, an inversion time of 400, and a 20 × 15 field of view, with 256 × 224/4 excitations to create 60 contiguous 1 mm coronal brain images. Image information was transferred to a computer using Canvas software (Deneba Software, Miami, FL). Measurements were made by overlaying and centering on the midline of each coronal MRI section a 1.0 mm2 matrix. The mediolateral, anteroposterior, and dorsoventral location of the amygdala in relation to the beads was determined. These measurements were used to plan the stereotactic coordinates for the ibotenic acid injection matrix.
With the exception of one animal, all animals received bilateral lesions simultaneously. Initially, the procedure was performed in two unilateral stages because of concerns about ibotenic acid toxicity. However, subsequent studies revealed that bilateral lesions could be safely performed in one surgery. To produce the lesions, the animals received isofluorane gas anesthesia. Heart rate, rectal temperature, and respiratory rate were monitored throughout the surgical procedure, and prophylactic doses of nafcillin (50 mg, i.v.) or cefazolin (20 mg/kg, i.m.) were given just before surgery. The animal was placed in a Kopf stereotaxic apparatus. Using standard aseptic surgical techniques, the skull was exposed; skull openings of 1.5 cm in diameter were made above the intended lesion sites. Stereotactic coordinates obtained from the MRI were refined using electrophysiological data obtained by recording cell firing from the cortical surface, the cell-rich zones of the amygdala, and the ventral surface of the brain.
One microliter of ibotenic acid was infused at a rate of 0.2 μl/min into 16–23 sites distributed over the entire volume of the amygdala on each side of the brain. During surgery, mannitol (1.5–2.0 gm/kg, i.v.) was administered over 30 min to control brain swelling. After the ibotenic acid injections were made, the midline incision was sutured, and the animal recovered from anesthesia. To ease postsurgical discomfort, buprenorphine (0.03 mg/kg, i.m.) and acetaminophen (20 mg/kg) were administered.
Histology and lesion verification. Lesioned animals were killed using methods consistent with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Animals were perfused with heparinized PBS and 4% paraformaldehyde. Histology was further processed by NeuroSciences Associates (Knoxville, TN). The brain slab containing the amygdala was cryoprotected in 2% DMSO and 20% glycerol for 12–18 hr, encased in a gelatin matrix after hardening in 10% buffered formalin, and then freeze-sectioned into 40-μm-thick slices. Every sixth section was collected in phosphate-buffered 10% formalin and stained with thionine. Sections through and beyond the anteroposterior extent of the amygdala were analyzed for tissue destruction using anatomical landmarks defined by Amaral et al. (1992). The percentage of the amygdala that was lesioned was determined by drawing the left and right amygdala lesion on four representative coronal templates that were 1–2 mm apart (templates of the rhesus amygdala provided by Dr. Elizabeth Murray, National Institute of Mental Health, Bethesda, MD). Using the Adobe Photoshop program, the number of pixels representing the lesion in each section was divided by the number of pixels representing the entire amygdala in that section. The sum of the pixels representing the lesion from each section was divided by the total number of pixels representing the complete amygdala. In addition, estimates were made of the percentage of destruction of the major nuclei (lateral, basolateral, accessory basal, and central).
Testing. When possible, behavioral tests and physiological data were collected before and after surgery. However, the animals rapidly habituate to many of the tests. Therefore, these tests could only be performed one time, which was after the surgery. All tests were performed in the same order for each animal, and postsurgical testing did not begin for at least 1 month after surgery. We have demonstrated previously that at the testing intervals used the monkeys do not habituate to the human intruder paradigm and the measurement of regional EEG. Therefore, these assessments were performed on the experimental animals before and after surgery and at the same intervals in the controls. Because animals rapidly habituate to exposure to the snake and a novel conspecific, the snake fear and social threat paradigms were performed only one time, which was after the surgery. Only six animals were used in the social threat and snake fear tests because a subset of the animals were killed early to verify lesions.
Human intruder paradigm. For testing, animals were placed in a cage that was 79 × 76 × 71 cm. The alone condition lasted for the first 10 min that the animal was in the cage by itself. After 10 min, a human entered the room and presented her profile to the monkey, standing 2.5 m from the cage while remaining motionless and avoiding any eye contact with the animal (NEC). The human left the room after 10 min and reentered the room 3 min later for the stare condition (ST). During ST, the intruder remained motionless 2.5 m from the cage while staring with a neutral face, directly at the animal. The human left the room 10 min later. Behavior and vocalizations were recorded on videotape. Extensively trained raters who were unaware of the treatment conditions performed the behavioral ratings using previously validated methods (Kalin and Shelton, 1989). The most prominent behaviors were freezing occurring during the NEC condition and defensive hostility induced by the ST condition. Freezing is defined by a period of at least 3 sec characterized by tense body posture, no vocalizations, and no movement other than slow movements of the head. Defensive hostility is characterized by any hostile behaviors directed at the tester, such as barking, head bobbing, open mouth threat face, and ear flapping.
Snake fear testing. Subjects were adapted to the Wisconsin General Testing Apparatus (WGTA) test cage for 1 hr on the first day. During the next 3 consecutive days of adaptation, the animals were each given 35 food items consisting of seven chocolate chips, seven plain M & Ms, seven Froot Loops, seven cocktail peanut halves, and seven raisins. The food items were randomly placed on top of a clear plastic stimulus presentation box (57.2 × 22.1 × 6.5 cm). Subjects remained in the test environment until either all 35 rewards were consumed or 1 hr elapsed. The order of retrieval of each food item was recorded to determine the two most preferred foods for each subject. The preferred items were used as rewards during testing.
On day 5 of adaptation, monkeys were presented with their two most preferred foods on top of the stimulus presentation box. This was done by opening the WGTA window for 60 sec, 24 times with a 45 sec interval between presentations. Food choices were presented in a random order with the preferred food of each subject randomly alternating between the left or right side of the top of the stimulus presentation box. Each subject was required to choose at least one food item within the allotted 60 sec. This criterion had to be met on at least 20 of the 24 presentations. Adaptation continued for additional days until this criterion was achieved.
To test for snake fear, monkeys were presented with the stimulus box that contained one of four stimuli: (1) nothing (empty box); (2) tape (8.8 cm diameter roll of blue masking tape); (3) fake snake (a curled black rubber snake 120 cm in length); and (4) snake [a live northern pine snake (Pithucus melanoleucusi)]. For each trial, the two most preferred foods were placed in the center and on top of the stimulus presentation box.
Each stimulus was presented six times during each test day in a pseudorandom order. Snake or fake snake stimuli were never presented during the first four trials of the first testing day, and no item from either the snake or the non-snake stimulus category was presented for more than three consecutive trials. All monkeys were presented with the same order of stimuli each day, with the order differing for each of the three testing days. Each trial lasted 60 sec regardless of the response of the subject, and the intertrial interval was 45 sec. Latencies to reach for the first and second food items were recorded.
The animals were observed to withdraw to the back of the cage when presented with the snake stimuli. Therefore, the number of times animals withdrew during each stimulus presentation was assessed by reviewing the videotapes of each stimulus trial.
Social threat paradigm. The test animal was placed in a large cage (151 × 71 × 68 cm) partitioned with a transparent plastic divider separating it from a threatening novel adult male rhesus monkey. Exposure to the threatening male lasted 1 hr; the behavioral responses were recorded on videotape for later scoring with a standard scoring system. Prominent behaviors occurring in the presence of the unfamiliar adult male included submit, fear grimace, coo, bark, locomotion, and environmental exploration. Submit is a combination of fearful and/or submissive behaviors such as crouching, fleeing, and withdrawal. Fear grimaces are characterized by a facial expression in which the corners of the mouth and lips are retracted, resulting in exposure of the teeth. Coo vocalizations are calls that increase and then decrease in frequency and intensity. Coos are made by rounding and pursing the lips. Bark vocalizations are made by forcing air from the abdomen through the vocal chords, producing a short, rasping, low-frequency sound. The definitions of locomotion and environmental exploration, as well as other behaviors, have been described previously (Kalin and Shelton, 1989).
EEG asymmetry. Regional EEG measures were obtained before and after surgery. To obtain regional EEG data, animals were manually restrained, and methods previously validated in rhesus monkeys were used (Davidson et al., 1992; Kalin et al., 1998). EEG measures were recorded and stored digitally according to methods described previously (Davidson et al., 1992).
All EEG data were reanalyzed to derive an EEG signal based on a computed average mastoids reference. A fast Fourier transform (FFT) was performed on all of the data selected in overlapping (50%) 2 sec chunks that were passed through a Hamming window to minimize end effects. Spectral power estimates from the FFT were averaged across chunks within stages of vigilance and power density (in square microvolts per Hertz) and were computed for the four bands: 1–4 Hz, 4–8 Hz, 8–12 Hz, and 13–30 Hz in each stage of vigilance. The 4–8 Hz band was chosen because robust lateralized changes have been seen in rhesus monkeys given diazepam (Davidson et al., 1992). Power density measurements were normalized by log transformation. The direction and magnitude of asymmetry were expressed as the log-transformed power density of an electrode position on the right side of the head less the log-transformed power density of the corresponding electrode on the left side of the head. ANOVA was performed on frontal and parietal asymmetry scores comparing different treatments.
Statistical analysis. Histological analysis revealed that nine animals had amygdala lesions that were characterized by a minimum of 70% damage. Four of these animals had lesions that were >95%. Eight animals had minimal lesions that varied greatly in percentage and region of destruction. Comparisons were made between animals with large lesions (>70%) and unoperated controls. In these animals, minimal damage was observed in regions surrounding the amygdala. Because of the heterogeneity between animals in these minimally damaged areas, no attempt was made to correlate extra-amygdala damage with behavioral and electrophysiological effects. To decrease the possibility that negative findings occurred because of incomplete lesions, additional comparisons were made between the control group and animals with >95% damage. Between-groups repeated-measures ANOVAs were used to analyze the behavioral and EEG data. Non-normally distributed data were log-transformed, and post hoc contrasts were used for subsequent comparisons. The Mann–Whitney U test was used to analyze the withdrawal responses occurring during snake exposure. Fisher's exact test was used to analyze the presence or absence of submissive behavior, fear grimacing, cooing, and barking occurring when animals were tested in the social threat paradigm. ANOVAs were performed on the duration of locomotion and environmental exploration occurring in response to the threatening adult male. Pearson product regression analyses were performed on the freezing, defensive hostility, and EEG asymmetry data to assess the effects of the lesions on the stability of individual differences in these parameters.
RESULTS
Extent of amygdala lesions
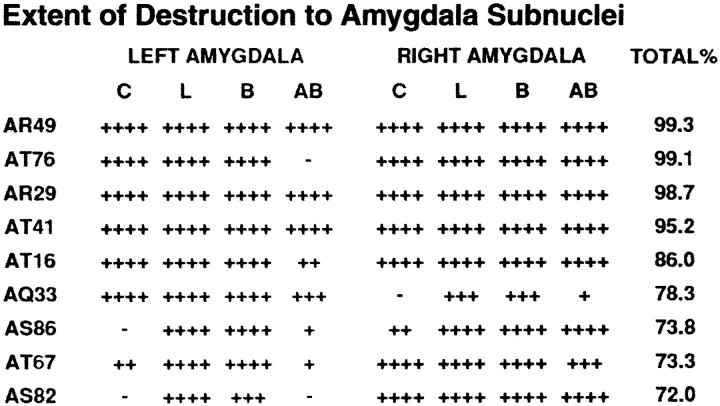
Of the 17 lesioned animals, nine (7 males and 2 females) had >70% lesions (72–99.3%), and four of these animals had >95% destruction. Eight of the 17 animals had minimal lesions with <70% destruction, ranging from 7 to 67%. To assess the effects of amygdala lesions on behavior and physiology, the animals with >70% lesions (the sample size for this group varied from six to nine depending on the dependent measure used) were compared with unoperated control animals (n = 10). Unoperated animals were used for comparison because we found that they did not differ from animals with the smallest lesions (<70%). Thus, the potential nonspecific effects of the surgical procedure were not significant factors. Figure1 compares the neuronal structure between an intact and lesioned amygdala. Figure 2displays the extent of the lesion in each of the nine animals with >70% amygdala destruction. Table1characterizes the extent of destruction in the central, lateral, basal, and accessory basal nuclei for each animal as well as the minimal damage to areas in close proximity to the amygdala. As can be seen in Figure 2, five animals had minimal damage to the anterior piriform cortex, four animals had minimal damage to the anterior portion of the entorhinal cortex, and two animals had minimal damage to the fundus of the superior temporal sulcus. A small portion of the dorsal claustrum was damaged in three animals, the tail of the caudate was minimally damaged in three animals, and two animals sustained minor damage to the posterior region of the bed nucleus of the stria terminalis. Eight of the animals had minimal damage to the anterior tip of the hippocampus.
Fig. 1.
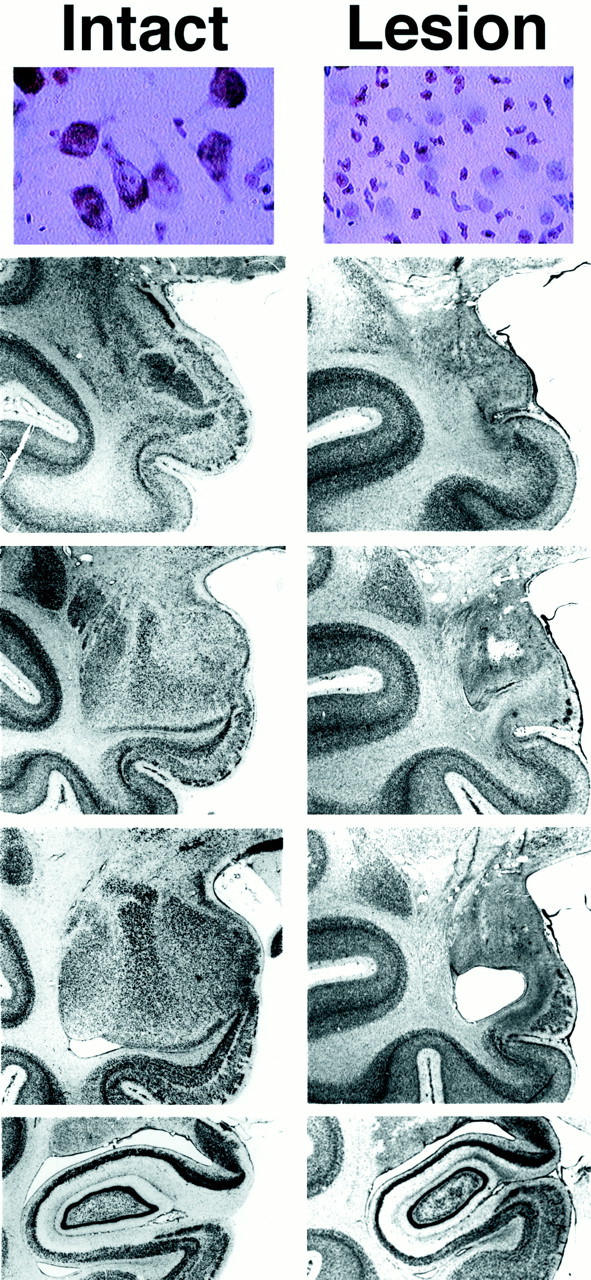
Top row, Magnified view (400×) of an ibotenic acid-lesioned and intact amygdala demonstrating a complete loss of neurons with infiltration of glial cells in the lesioned animal. Bottom rows, Coronal sections (magnification 4.5×) through the anteroposterior (topto bottom) extent of the amygdala and anterior hippocampus of a control and AT41 (95.2% amygdala damage). Thefirst column displays an intact left amygdala and thesecond column displays the left amygdala of AT41. Note that AT41 has a complete lesion with tissue shrinkage in the amygdala region with no damage to the hippocampus.
Fig. 2.
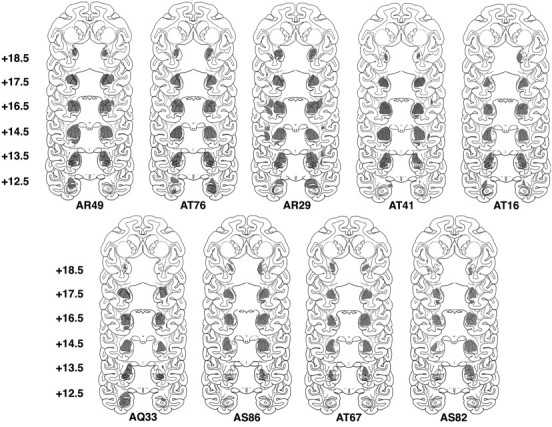
Extent of the lesions displayed on standardized templates from the nine animals with >70% bilateral amygdala lesions that are described in Table 1. In relation to the interaural line, coronal sections are arranged top tobottom from 18.5 to 12.5 mm, anteroposterior.
Table 1.
The total and percent destruction of central (C), lateral (L), basal (B), and accessory basal (AB) nuclei for the nine animals with >70% ibotenic acid lesions. Percentage of destruction for each nucleus is indicated as follows: −, No destruction; +, up to 25%; ++, 26–50%; +++, 51–75%; ++++, 76–100%.
Effects of amygdala lesions on snake fear
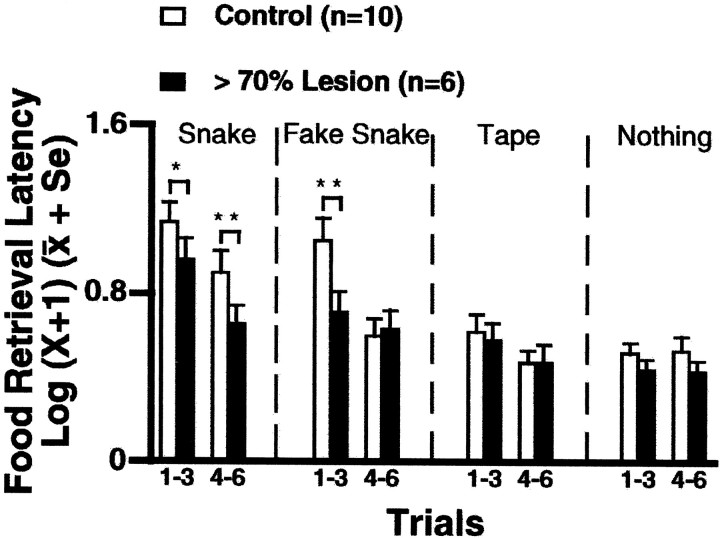
A between-groups repeated-measures ANOVA demonstrated a main effect of stimulus (F = 24.17; df = 3,14;p < 0.0001). The mean latency for treat retrieval in the presence of the real snake was greater than that for the fake snake. Both snake conditions induced greater retrieval latencies than did the tape condition, and retrieval latencies for the tape and nothing conditions did not differ. A significant lesion × stimulus × trial interaction was also evident (F= 3.29; df = 3,42; p < 0.03). Compared with controls, lesioned animals (n = 6) had significantly shorter retrieval latencies when exposed to the real snake during trials 1–6 and in the presence of the fake snake during trials 1–3 (Fig. 3). It is important to note that the lesions reduced the effects of the snake stimuli on the retrieval latency but did not completely block the response. Examination of the data from the individual trials revealed that the initial response to the snake was reduced in the lesioned animals compared with the controls. Both control and lesioned animals displayed a similar pattern of habituation.
Fig. 3.
Effects of >70% lesions (solid bars; n = 6) compared with controls (open bars; n = 10) on the latency to retrieve a treat in the presence of a real snake, fake snake, roll of tape, and nothing. Across all trials, lesioned animals more rapidly retrieved a treat placed above the real snake than did control animals. This effect was also observed in the presence of the fake snake during the first three trials (*p < 0.05; **p < 0.01). No significant differences in retrieval latency between lesioned and control animals were found in response to the roll of tape or nothing.
Mann–Whitney U tests revealed that control animals withdrew to the back of the cage significantly more often in the presence of the snake stimuli compared with the roll of tape (p< 0.05). In addition, the control animals engaged in more withdrawal behavior than did the lesioned animals in the presence of the snake (trials 1–3, p < 0.025; trials 4–6,p < 0.05).
Social threat paradigm
Fear-related responses that occurred because of the presence of the threatening unfamiliar adult male monkey included fear grimacing and submissive behaviors. As summarized in Table2, the lesioned animals (n = 6) engaged in less submissive behavior (p < 0.04) and emitted fewer fear grimaces (p < 0.02) than control animals. In addition, the lesioned animals emitted significantly fewer coo (p < 0.008) and bark (p< 0.04) vocalizations in the presence of the threatening adult male. The amount of locomotion and environmental exploration did not significantly differ between the lesioned and control animals.
Table 2.
Behavioral responses to social threat
| No. of control subjects (n = 10) | No. of lesion subjects (n = 6) | p < | |
|---|---|---|---|
| Fear grimace | 7 | 0 | 0.02 |
| Submit | 9 | 2 | 0.04 |
| Coo | 9 | 1 | 0.01 |
| Bark | 7 | 0 | 0.04 |
The effects of the amygdala lesions on behaviors expressed in the social threat paradigm are displayed. Significantly fewer subjects in the >70% group engaged in fearful and defensive behaviors in response to the threatening adult male than did controls (n = 10) as determined by Fisher's exact test.
Defensive responses in the human intruder paradigm
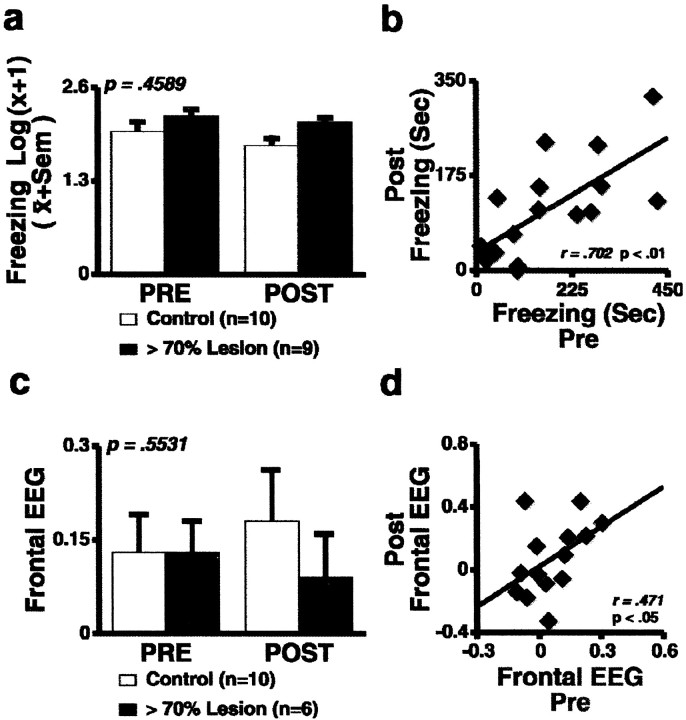
Lesioned monkeys (n = 9) were tested twice before and twice after amygdala lesions; control animals were tested repeatedly at the same intervals. Across both groups, a main effect of test condition was seen such that NEC induced the greatest amount of freezing (F = 16.93; df = 3,17; p< 0.0001) and ST induced the most defensive hostility (F = 59.55; df = 1,17; p < 0.0001). There were no significant differences between lesion and control animals with regard to NEC-induced freezing (F= 0.58; df = 1,17; p = 0.459) (Fig.4a) or ST-induced hostility (F = 0.39, df = 1,17; p = 0.541) when compared before and after surgery. To exclude the possibility that this lack of difference was because of incomplete lesions, the subset of four animals with lesions >95% was compared with controls. No significant difference was found for NEC-induced freezing when the animals with lesions >95% were compared with controls before and after surgery (F = 0.15; df = 3,36;p = 0.928). For all of the lesioned animals tested (n = 17), individual differences in freezing and defensive hostility before surgery were significantly correlated with those after surgery (r = 0.702 and 0.683, respectively;p values < 0.01) (Fig. 4b). For only those animals with >70% lesions, the correlation remained positive for freezing (r = 0.31) but lost significance. For hostility, the correlation for only those animals with >70% lesions was r = 0.924 (p < 0.001).
Fig. 4.
a, Mean NEC-induced freezing for animals before and after and lesioning. Freezing was not affected by the amygdala lesions (solid bars, n= 9; open bars, controls, n = 10).b, In the entire group of lesioned animals (≤70%; n = 17), the stability of individual differences in NEC-induced freezing was unaffected by the lesions. c, Mean frontal EEG asymmetric electrical activity (log right to log left, 4–8 Hz band) was not changed by amygdala lesions (lesion group, n = 6, solid bars; controls, n = 10, open bars). d, Individual differences in frontal asymmetry remained stable in all animals that received lesions (n = 14).
Asymmetric frontal electrical activity
Asymmetric frontal EEG patterns did not significantly differ between control and lesion animals before and after surgery (F = 0.37; df = 1,14; p = 0.553) (Fig. 4c). Additional evidence that the amygdala lesions did not affect frontal EEG asymmetry is provided by the significant correlation between EEG asymmetry before and after surgery within all 14 lesioned animals in which EEG was measured (r = 0.471; p < 0.05; one-tailed t test) (Fig. 4d). The correlation for the six animals tested with >70% lesions was also significant (r = 0.878;p < 0.05). These correlations are similar in magnitude to those observed in other studies when regional EEG measures were repeatedly assessed in the same animals (Kalin et al., 1998).
DISCUSSION
These data are among the first using selective fiber-sparing excitotoxic lesions to study the role of the primate amygdala in emotional processing. The data demonstrate a role of the amygdala in mediating acute unconditioned fear responses to stimuli with which the monkeys had no previous experience (a snake and an unfamiliar adult male monkey). In addition, in a subset of these animals, we found that amygdala lesions blocked the effects of acute unconditioned stress on disrupting sleep (Benca et al., 2000). In contrast, human intruder-induced defensive freezing and hostility, behaviors that represent unconditioned responses that are present early in life and characterize the long-term emotional disposition of a monkey, remained unaffected by the amygdala lesions. In addition, the amygdala does not appear to be involved in maintaining individual differences in freezing behavior and asymmetric frontal electrical activity. The lack of effect of amygdala lesions on trait-like defensive responses produced by exposure to a human intruder is noteworthy because early studies in primates reported that amygdala lesions resulted in marked personality changes, in a “taming effect,” and in other changes such as hyperorality and excessive exploratory behavior (Kluver and Bucy, 1939;Weiskrantz, 1956). However, these studies were based on lesions that nonselectively damaged cells and fibers of passage in the amygdala, as well as in other regions of temporal cortex. Data from three rhesus monkeys with more selective but total electrolytic lesions of the amygdala demonstrated effects similar to the earlier studies (Aggleton and Passingham, 1981). Interestingly, Zola-Morgan et al. (1991) reported a lack of effect of total and selective radiofrequency amygdala lesions in three cynomologous monkeys (Macaca fasicularis) on fearful responses induced by social stimuli. Data from a very recent study in rhesus monkeys demonstrated that ibotenic acid lesions attenuated a number of fear-related responses elicited by the presentation of social and inanimate stimuli. The ibotenic acid lesions reduced freezing in response to snake exposure but had marginal effects on freezing and other defensive behaviors induced by brief exposure to human faces. Effects on these responses were much more robust in animals with aspiration lesions of the amygdala (Meunier et al., 1999). This direct comparison of the effects of aspiration and ibotenic acid lesions in primates suggests that many of the more dramatic responses previously ascribed to amygdala lesions occur because of damaging fibers of passage coursing through the amygdala.
Studies in rodents demonstrate that excitotoxic amygdala lesions can disrupt the acquisition and expression of conditioned freezing responses (Ledoux et al., 1988; Davis, 1992). These findings might appear to be inconsistent with the lack of effect that we observed on defensive freezing in the primates. However, it is important to note that the defensive freezing induced by the human intruder reflects an unconditioned response. Based on our results, we believe that it is not freezing per se that is important, but rather the specifics of the stimulus–response relationship such as the eliciting stimulus, the duration of stimulus presentation, and the amount of previous exposure as well as the length of time the animal has been responding to the stimulus before testing. In the human intruder paradigm, the unconditioned freezing responses of the monkeys as a result of the human intruder are present by 3 months of age (Kalin et al., 1991), and individual differences in this response remain stable as monkeys mature (Kalin and Shelton, 1989). In addition, laboratory monkeys are exposed to human faces daily such that before the human intruder tests they had considerable exposure to the eliciting stimuli. In the human intruder paradigm, the monkeys are tested with a prolonged (10 min) exposure to the human, whereas in the rodent studies that have examined the role of the amygdala in mediating conditioned freezing, freezing responses are tested shortly after they are learned. Other rodent studies suggest a diminished effect of amygdala lesions in animals that have had significant previous experience with the stimuli (Parent et al., 1992). Rodent studies also demonstrate a role of the amygdala in the storage of emotional memories, but a functional amygdala is not necessary for the expression of behaviors associated with retained memories (McGaugh et al., 1996). In addition, studies in rodents support the concept that the amygdala is involved in mediating fear, but other structures such as the bed nucleus of the stria terminalis may be important in mediating more long-term, nonspecific anxiety responses (Davis et al., 1997).
The central nucleus is a site that is important in the expression of fear-related responses (Ledoux et al., 1988; Davis, 1992). It could be argued that the lack of effect on freezing observed in the current study could be because some of the animals in this study had only partial damage to the central nucleus. However, freezing and defensive hostile responses were intact in the five animals (AR49, AT76, AR29, AT41, and AT16) with unequivocal complete bilateral central nucleus damage. The possibility exists that in primates, prefrontal cortical regions, such as the orbitofrontal cortex, may play a role in mediating responses elicited in the human intruder paradigm that are associated with anxious temperament. In primates, data suggest that the orbitofrontal cortex is associated with guiding behavior based on an appraisal of its future positive and negative consequences (Bechara et al., 1994) and may have a role in regulating the temporal aspects of behavioral and emotional responses. In addition, there are prominent bidirectional linkages between the orbitofrontal cortex and the amygdala (Amaral et al., 1992). A role for the orbitofrontal cortex is also consistent with our finding that animals with extreme right frontal cortical electrical activity are dispositionally anxious and fearful (Kalin et al., 1998). Our data suggest that if the orbitofrontal cortex is involved in mediating the anxious temperament endophenotype, input from the amygdala is not critical. It is possible that other prefrontal regions and/or other brain regions such as the bed nucleus of the stria terminalis are also involved. Human studies examining the effects of relatively selective amygdala lesions demonstrate a role of the amygdala region in the recognition of fearful facial expressions and in acquiring conditioned autonomic responses (Adolphs et al., 1994; Bechara et al., 1995). However, such studies have not examined the effect of the lesion on anxious temperament because such measures have never been obtained before lesion.
According to previous studies together with the data in this study, we believe that in primates the amygdala has an important role in mediating initial responses to fearful stimuli. This is consistent with the demonstration of rapid amygdala habituation in humans exposed to fearful stimuli (Breiter et al., 1996) and is consistent with the concept that a primary role of the amygdala is to process novelty and ambiguity related to potentially threatening situations (Whalen, 1998). However, we believe that fear-related or anxiety responses characteristic of temperament, stable and present from early in life, are not mediated by the amygdala. Furthermore, physiological responses, such as frontal EEG asymmetry, that are associated with these behaviors are unaffected by amygdala lesions. Although our data suggest that the amygdala is not critical in maintaining responses associated with the anxious endophenotype, it is possible that the amygdala plays a developmental role early in life in the acquisition and expression of these responses. Data from monkeys that underwent large medial temporal lobe ablations or more selective hippocampal lesions demonstrate differential effects on social behavior depending on the age at which the lesions were made (Malkova et al., 1997; Bachevalier et al., 1999). For example, marked effects on adult social behavior occurred when animals were lesioned as infants. When lesions were made in adults, the effects on social behavior were much less prominent. Similar data exist in the human literature. Humans with congenital Urbach–Wiethe disease demonstrate bilateral amygdala damage that is thought to manifest itself in childhood. These individuals are impaired in their ability to recognize fearful facial expressions. In contrast, individuals who have acquired amygdala damage well into their adulthood are not reported to be impaired in their ability to recognize fearful facial expressions (Hamann et al., 1996).
We suggest that prefrontal cortical regions are important in mediating the behaviors associated with anxious temperament because an association exists between patterns of prefrontal asymmetric electrical activity and temperamental features and because these asymmetric patterns of frontal activity are unaffected by amygdala lesions.
Footnotes
This work was supported in part by Grants MH46729, MH52354, MH18931, and MH61083, by the Health Emotions Research Institute, and by Meriter Hospital. We thank H. VanValkenberg, K. Lee, L. MacDonald, D. Makuch, C. Quanbeck, D. Amaral, R. Benca, S. Zimbric, I. Dolski, and A. Skolnick. We also thank the staff at the Harlow Center for Biological Psychology and the Wisconsin Regional Primate Research Center for their technical support.
Correspondence should be addressed to Ned H. Kalin, Department of Psychiatry, 6001 Research Park Boulevard, Madison, WI 53719. E-mail:nkalin@facstaff.wisc.edu.
REFERENCES
- 1.Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- 2.Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta). J Comp Physiol Psychol. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- 3.Amaral DG, Price JL. An air pressure system for the injection of tracer substances into the brain. J Neurosci Methods. 1983;9:35–43. doi: 10.1016/0165-0270(83)90107-3. [DOI] [PubMed] [Google Scholar]
- 4.Amaral DG, Price JL, Pitkanen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The amygdala. Wiley-Liss; New York: 1992. pp. 1–67. [Google Scholar]
- 5.Bachevalier J, Alvarado MC, Malkova L. Memory and socioemotional behavior in monkeys after hippocampal damage incurred in infancy or in adulthood. Biol Psychiatry. 1999;46:329–339. doi: 10.1016/s0006-3223(99)00123-7. [DOI] [PubMed] [Google Scholar]
- 6.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;40:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 7.Bechara A, Tranel D, Damasio H, Adolphs R, Rockland C, Damasio AR. Double dissociation of conditioning and declarative knowledge relative to the amygdala and hippocampus in humans. Science. 1995;269:1115–1118. doi: 10.1126/science.7652558. [DOI] [PubMed] [Google Scholar]
- 8.Benca RM, Obermeyer WH, Shelton SE, Droster J, Kalin NH. Effects of amygdala lesions on sleep in rhesus monkeys. Brain Res. 2000;879:130–138. doi: 10.1016/s0006-8993(00)02761-x. [DOI] [PubMed] [Google Scholar]
- 9.Biederman J, Chaloff J, Hirshfeld DR, Kagan J. A 3-year follow-up of children with and without behavioral inhibition. J Am Acad Child Adolesc Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J Comp Physiol Psychol. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- 11.Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- 12.Davidson RJ. Cerebral asymmetry, emotion, and affective style. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. MIT; Cambridge, MA: 1995. pp. 361–387. [Google Scholar]
- 13.Davidson RJ, Irwin W. The functional neuroanatomy of emotion and affective style. Trends Cogn Sci. 1999;3:11–21. doi: 10.1016/s1364-6613(98)01265-0. [DOI] [PubMed] [Google Scholar]
- 14.Davidson RJ, Kalin NH, Shelton SE. Lateralized effects of diazepam on frontal brain electrical asymmetries in rhesus monkeys. Biol Psychiatry. 1992;32:438–451. doi: 10.1016/0006-3223(92)90131-i. [DOI] [PubMed] [Google Scholar]
- 15.Davis M. The role of the amygdala in conditioned fear. In: Aggleton J, editor. The amygdala. Wiley-Liss; New York: 1992. pp. 255–305. [Google Scholar]
- 16.Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos Trans R Soc Lond B Biol Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drevets WC, Raichle ME. Positron emission tomographic imaging studies of human emotional disorders. In: Gazzaniga MS, editor. The cognitive neurosciences. MIT; Cambridge, MA: 1995. pp. 1153–1164. [Google Scholar]
- 18.Hamann SB, Stefanacci L, Squire L, Adolphs R, Tranel D, Damasio H, Damasio A. Recognizing facial emotion. Nature. 1996;379:497. doi: 10.1038/379497a0. [DOI] [PubMed] [Google Scholar]
- 19.Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- 20.Kalin NH. The neurobiology of fear. Sci Am. 1993;268:94–101. doi: 10.1038/scientificamerican0593-94. [DOI] [PubMed] [Google Scholar]
- 21.Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- 22.Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: ontogeny and context-dependent selective expression. Child Dev. 1991;62:1175–1183. [PubMed] [Google Scholar]
- 23.Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperaments in rhesus monkeys. Behav Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- 24.Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- 25.Kling A, Brothers LA. The amygdala and social behavior. In: Aggleton J, editor. The amygdala. Wiley-Liss; New York: 1992. pp. 353–377. [Google Scholar]
- 26.Kluver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Arch Neurol Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- 27.Ledoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malkova L, Mishkin M, Suomi SJ, Bachevalier J. Socioemotional behavior in adult rhesus monkeys after early versus late lesions of the medial temporal lobe. Ann NY Acad Sci. 1997;807:538–540. doi: 10.1111/j.1749-6632.1997.tb51961.x. [DOI] [PubMed] [Google Scholar]
- 29.McGaugh JL, Cahill L, Roozendaal B. Involvement of the amygdala in memory storage: interactions with other brain systems. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. Eur J Neurosci. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- 31.Murray EA, Mishkin M. Object recognition and location memory in monkeys with excitotoxic lesions of the amygdala and hippocampus. J Neurosci. 1998;18:6568–6582. doi: 10.1523/JNEUROSCI.18-16-06568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parent MB, Tomaz C, McGaugh JL. Increased training in an aversively motivated task attenuates the memory-impairing effects of posttraining N-methyl-d-aspartate-induced amygdala lesions. Behav Neurosci. 1992;106:789–797. doi: 10.1037//0735-7044.106.5.789. [DOI] [PubMed] [Google Scholar]
- 33.Weiskrantz L. Behavioral changes associated with ablation of the amygdaloid complex in monkeys. J Comp Physiol Psychol. 1956;4:381–391. doi: 10.1037/h0088009. [DOI] [PubMed] [Google Scholar]
- 34.Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr Dir Psychol Sci. 1998;7:177–188. [Google Scholar]
- 35.Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]