Abstract
Most of the research on ventral striatal functions has been focused on their role in modulating reward and motivation. More recently, a possible role of this structure in cognitive functions has been suggested. However, very little information is available on the involvement of the nucleus accumbens in the different stages of the consolidation process. In this study, the effect of focal injections of AP-5 and DNQX, competitive antagonists at the NMDA and AMPA receptors, respectively, was examined in a nonassociative task designed to estimate the ability of mice to react to spatial changes. The task consists of placing the animals in an open field containing five objects; after three sessions of habituation, their reactivity to object displacement was examined 24 hr later.
AP-5 injections administered after training impaired the ability of mice to detect the spatial novelty but did not affect response when injected 120 min after training or before testing. On the contrary, DNQX did not affect response when administered immediately or 120 min after training but did impair spatial discrimination when administered before training or testing. These data demonstrate a double dissociation between glutamate receptor subtypes, such that accumbens NMDA receptors are important for consolidation and not ongoing discrimination of spatial information, whereas AMPA receptors have an opposite role in these processes.
Keywords: nucleus accumbens, glutamate, consolidation, spatial learning, DNQX, AP-5
Most of the research on the functions of the nucleus accumbens (Nacc) has been focused on its role in modulating reward and motivation (Mogenson, 1987). However, more recently a possible role of the basal ganglia in modulating cognitive functions has been emphasized (Graybiel, 1995; White, 1997). Based on the reciprocal neuroanatomical relationships between the cortex and striatum, it has been suggested that the striatal complex, including the dorsal and ventral striatum, might play a substantial role in processing information relayed from the cortex.
In particular, there is evidence of a possible role of the ventral striatum in processing spatial information (Annett et al., 1989;Seamans and Phillips, 1994; Maldonado-Irizarry and Kelley, 1995;Floresco et al., 1996, 1997; Roullet et al., 1997; Usiello et al., 1998). This hypothesis is sustained by neuroanatomical data that show dense projections to the Nacc from structures involved in spatial learning, such as the hippocampus, the prefrontal cortex, and the entorhinal cortex (Beckstead, 1979; Kelley and Domesick, 1982;Groenewegen et al., 1987). Analyses of the effects of selective lesions to the main sources of limbic glutamatergic afferent to the Nacc and of disconnecting procedures suggest that spatial information processing in this structure is dependent on intact hippocampal and prefrontal cortex transmission (Schacter et al., 1989; Roullet et al., 1997; Sargolini et al., 1999).
Limbic projections to the accumbens are thought to be mainly glutamatergic (Walaas and Fonnum, 1979), and high density of NMDA as well as AMPA receptors has been reported in this structure (Albin et al., 1991). If spatial information from different limbic structures is relayed in the accumbens, blockade of NMDA and AMPA receptors should impair the response in spatial tasks. Along this line, it has been demonstrated that focal administration of AMPA and NMDA antagonists into the Nacc induced a specific deficit in spatial learning tasks (Maldonado-Irizarry and Kelley, 1995; Sargolini et al., 1999). This evidence strongly suggests an involvement of glutamate receptors located in the Nacc in processing spatial information. Pretraining manipulations affect different stages of memorization; therefore, it is difficult from these studies to determine the phase of memory formation in which the Nacc is involved. Furthermore, the Nacc has been demonstrated to play a major role in mediating reward-related learning (Robbins and Everitt, 1996). Pharmacological manipulations of this structure could therefore affect reward-related processes rather then plastic changes necessary for spatial information storage.
The purpose of this study was to investigate the possible involvement of NMDA and non-NMDA receptors located into the Nacc in the different aspects of spatial learning. The task we chose is a nonassociative task in which no explicit reward was present and in which no stimulus response association was required. It consists of placing mice in an open field containing five objects and, after three sessions of habituation, examining their reactivity to object displacement. Control animals usually show an increased exploration of the displaced object; this response is usually interpreted as an index of the ability of animals to detect and react to the spatial change (Poucet, 1989;Thinus-Blanc et al., 1992).
MATERIALS AND METHODS
Animals. The subjects were CD1 outbred mice obtained from Charles River (Calco, Italy). At the time of surgery, they were ∼9- to 10-weeks-old, and their weights ranged from 35 to 40 gm. On arrival, mice were housed in groups of five in standard breeding cages (21 × 21 × 12 cm) placed in a rearing room at a constant temperature (22 ± 1°C) with food and water available ad libitum. They were tested during the first half of the light period (between 9:00 A.M. and 2:00 P.M.).
Every possible effort was made to minimize animal suffering, and all procedures were in strict accordance with European community and Italian national laws and regulations on the use of animals in research and National Institutes of Health guidelines on animal care.
Surgery. Mice were anesthetized with chloral hydrate (400 mg/kg) and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) with mouse adapter and lateral bars. After placing the animals on the stereotaxic apparatus, the head skin was cut longitudinally and bilateral guide cannulae (0.5 mm in diameter), aimed at the core of the accumbens, were fixed on the calvarium with dental acrylic. The following coordinates with lambda and bregma in the same horizontal plane were used: anterior to bregma, +1.7 mm; lateral to midline, ±1 mm; ventral from the dura, −2 mm, according to Franklin and Paxinos (1997). The subjects were then left in their home cages for a recovery period of 7–10 d.
Reaction to novelty apparatus. The apparatus (Fig.1) was a circular open field that was 60 cm in diameter with a 30-cm-high wall made of gray plastic material and a floor painted white and divided into sectors by black lines. The open field was placed into a soundproof cubicle and surrounded by a visually uniform environment except for a conspicuous striped pattern that was 30-cm-wide and 36-cm-high (alternating 1.5-cm-wide vertical white and black bars) attached to the wall of the field. The apparatus was illuminated by a red light (80 W) located on the ceiling. A video camera above the field was connected to a video recorder and a monitor.
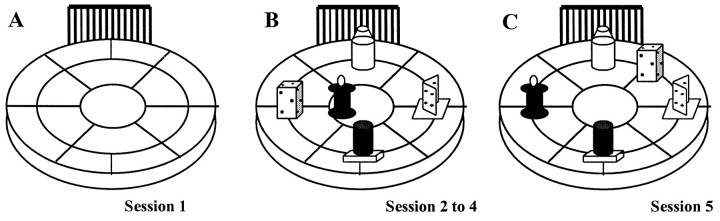
Fig. 1.
Schematic representation of the apparatus and the object configuration over successive sessions. A, The open field is initially empty (S1); in the three subsequent sessions (S2–S4), it is filled with objects in a particular configuration (B). In S5, two objects are displaced (spatial novelty) (C).
Five objects were simultaneously present in the open field: (1) a chromium-plated parallelepiped (7 × 4 × 4 cm) with holes irregularly distributed on the sides and the top; (2) a plastic cone on a transparent cylinder base (diameter of the section, 8 cm; height, 6 cm); (3) two gray iron rectangles (10 × 3 cm) forming a 90° angle, inserted on a rectangular plastic base (7 × 5 cm); (4) a black Plexiglas cylinder (height, 10 cm; diameter, 5 cm); and a (5) a black and gray spool (height, 9 cm; diameter of the top, 5 cm) on a square base (6 × 6 cm). The initial arrangement was a square with a central object (object E) as schematized in Figure 1.
Procedure. The first day, mice were individually submitted to four successive 6 min sessions, separated by a 3 min delay, during which the subjects were returned to their home cages. During session 1 (S1), the mice were placed into the empty open field to familiarize the animal with the apparatus and to record the baseline level of locomotor activity. During S2–S4, the objects were placed as shown in Figure1.
On the second day (24 hr later), mice were submitted to only one 6 min session, with the objects displaced relative to the original arrangement (S5). The configuration was changed by moving two objects: object E replaced object A, which was itself displaced at the periphery of the apparatus so that the initial square arrangement was changed to a polygon-shaped arrangement.
Drug injections were performed at different time points: for experiment 1, the NMDA antagonist, (−)-AP-5 (AP-5), was administered immediately after the last training session (S4) (n = 13, n = 8, n = 8, and n = 8, respectively, for saline and for AP-5 at 0.075, 0.15, and 0.3 μg/side); for experiment 2, the AMPA antagonist DNQX was injected immediately after the last training session (S4) (n = 7, n = 7, and n = 8, respectively, for saline and for DNQX at 0.001 and 0.005 μg/side); for experiment 3, AP-5 and DNQX were administered 120 min after S4 (n = 9, n = 8, and n = 9, respectively, for vehicle, AP-5 at 0.3 μg/side, and DNQX at 0.005 μg/side); for experiment 4, saline (n = 8), AP-5 (0.3 μg/side, n = 8), and DNQX (0.005 μg/side,n = 7) were administered 10 min before S5 on day 2; for experiment 5, vehicle (n = 7) and DNQX (0.001 μg/side, n = 7) were administered 10 min before S1.
The rationale for using these doses was based on previous studies and preliminary experiments in which they were demonstrated to be effective, when focally administered into the Nacc, in impairing spatial learning in the same task (Sargolini et al., 1999; F. Sargolini and A. Mele, unpublished observation). AP-5 was dissolved in saline solution, and DNQX was dissolved in a solution of 2% DMSO in distilled water. Both drugs were injected in a volume of 0.2 μl/side. The injection time was 2 min, and the needle was left in place for an additional 30 sec to allow diffusion. All mice were habituated to the injection procedure. Drug-injected animals were always compared with control mice injected with the same volume of vehicle solution. Because saline and DMSO vehicle-injected mice did not show any difference in experiments 1 and 2, control mice injected with saline and DMSO were pulled together in experiments 3 and 4. The injections were made by inserting an injection needle (0.25 mm in diameter) into the guide cannula, connected with polyethylene tubing to a 1 μl Hamilton syringe. During the injection time, the mice were returned to their cages.
Data collection and statistics. Data collection was performed using video recordings; the observer was always blind to treatment (Save et al., 1992). In the first session, locomotor activity was recorded by counting the number of sectors crossed by each animal while moving in the open field. From S2 to S5, object exploration was evaluated by the time spent by the animal in contact with an object. A contact was defined as the snout of the subject actually touching an object. The duration of contact is expressed as mean per object. In S5, the spatial arrangement of the objects was modified; response to spatial change was assessed by comparing the mean time in contact with the objects belonging to each category (displaced and nondisplaced) in S5 minus the mean time spent in contact with the same object category in S4.
Statistical analyses were performed using an ANOVA with a within-between design. For the habituation, the repeated factor was session (three levels: S2, S3, S4) and the between factor was treatment (levels in experiment 1 were 0.0, 0.075, 0.15, and 0.3 of AP-5; levels in experiment 2 were 0.0, 0.001, and 0.005 of DNQX; levels in experiments 3 and 4 were controls, AP-5, and DNQX; levels in experiment 5 were controls and DNQX). The exploration of the different object categories in S4 and S5 expressed as absolute values was analyzed by repeated two-factors treatment [sessions (two levels), S4 and S5; object category (two levels), displaced objects (DO), and nondisplaced objects (NDO)] and between factor treatment (same levels than before). The re-exploration of the different object categories expressed as exploration in S5–S4 for each object category was analyzed using a within (object category, two levels) and between (treatment) design. Tukey honestly significant difference and/or simple effects post hoc comparison were used when appropriate.
Cannula placement verification. At the completion of the experiment, mice were killed by an overdose of chloral hydrate; brains were removed and frozen at −20°C. Cannula placements were determined by examination of serial coronal sections (25 μm) stained with cresyl violet. Only animals showing a correct placement of the cannula were included in the statistical analysis.
RESULTS
Cannula placement verification
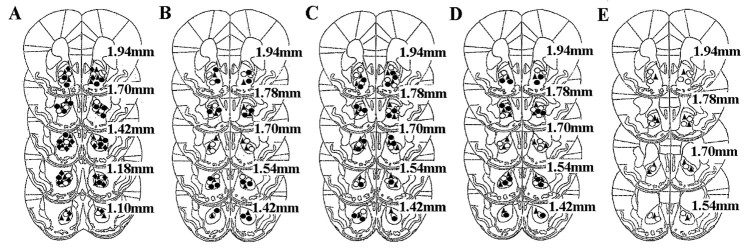
Figure 2 shows a schematic representation of the cannula placements for all of the experiments. Histological analysis shows that the injection site is located in the Nacc core for the majority of mice. Only animals showing correct Nacc placements were included in the statistical analysis.
Fig. 2.
Drawing of coronal sections from animals in all experiments. Each symbol represents the cannula placement.A, Immediate post-training AP-5 administrations.Filled triangles, saline; open circles, AP-5 at 0.07 μg/side; filled circles, AP-5 at 0.15 μg/side; filled diamonds, AP-5 at 0.3 μg/side.B, Immediate post-training DNQX administrations.Filled triangles, vehicle; open andfilled circles, respectively, AMPA antagonist at 0.001 and 0.005 μg/side. C, Cannula placements for administrations of vehicle (filled triangles), AP-5 at 0.03 μg/side (open circles), and DNQX at 0.005 μg/side (filled circles) 120 min after training. D, Cannula placements for mice administered vehicle (filled triangles), AP-5 at 0.15 μg/side (open circles), and DNQX at 0.001 μg/side (filled circles) before testing.E, Cannula placements for mice administered vehicle (filled triangles) and DNQX at 0.001 μg/side (open circles) before training.
Habituation
Table 1 shows the mean time of contact in S2, S3, and S4 with all five objects for each experiment. No major differences among groups were observed with regard to overall object exploration in S2, S3, and S4 when drugs were administered after training in experiments 1–4. Moreover, all groups showed a similar decrease in the time spent exploring the objects over sessions. The ANOVA revealed only a significant session effect but no drug effect and no session–drug interaction. Pretraining focal administrations of DNQX reduced the overall time spent by the animals exploring the objects only in experiment 5. It is worthwhile to note, however, that AMPA receptor blockade did not affect the habituation pattern of the mice. The ANOVA revealed a significant effect of drug (F(1,12) = 15.2; p = 0.0021) and sessions (F(2,24) = 36.5;p = 0.0001) but no interaction (F(2,24) = 0.14; p = 0.86). Table 1 shows the mean time of contact with the two object categories, DO and NDO, before spatial change in S4; control as well as drug-injected groups explored the two categories for a similar amount of time.
Table 1.
Mean duration of contact (± SEM) with the objects before and after spatial change in the different experimental groups
| Experiment | Groups (N) | Habituation | S4 | S5 | ||||
|---|---|---|---|---|---|---|---|---|
| S2 | S3 | S4 | DO | NDO | DO | NDO | ||
| 1 | Control (13) | 26.6 ± 1.5 | 19.03 ± 1.2 | 11.2 ± 1.1 | 10.0 ± 1.2 | 11.7 ± 1.3 | 15.9 ± 1.1* | 7.6 ± 0.78 |
| AP-5 0.075 (8) | 26.8 ± 2.3 | 14.9 ± 1.1 | 9.2 ± 0.8 | 8.7 ± 0.8 | 9.6 ± 0.9 | 12.6 ± 1.1*† | 6.8 ± 0.9 | |
| AP-5 0.15 (8) | 24.1 ± 1.4 | 15.9 ± 1.9 | 11.4 ± 1.5 | 11.3 ± 1.6 | 11.4 ± 1.5 | 10.6 ± 1.8† | 9.1 ± 0.9 | |
| AP-5 0.3 (8) | 30.7 ± 1.9 | 18.6 ± 1.3 | 12.4 ± 1.2 | 12.4 ± 1.4 | 12.4 ± 1.4 | 11.7 ± 1.6† | 11.4 ± 1.71-160 | |
| 2 | Control (7) | 23.1 ± 2.6 | 16.3 ± 1.5 | 11.2 ± 1.6 | 10.7 ± 1.6 | 11.5 ± 1.6 | 15.5 ± 2.1* | 7.9 ± 0.8 |
| DNQX 0.001 (7) | 24.9 ± 1.3 | 17.5 ± 1.5 | 10.5 ± 0.9 | 10.0 ± 1.2 | 10.9 ± 0.8 | 16.2 ± 1.6* | 7.8 ± 1.4 | |
| DNQX 0.005 (8) | 25.0 ± 0.8 | 14.7 ± 1.8 | 10.1 ± 1.4 | 10.4 ± 1.7 | 9.8 ± 1.5 | 16.2 ± 1.2* | 8.6 ± 0.6 | |
| 3 | Control (9) | 24.1 ± 1.2 | 13.6 ± 1.7 | 8.1 ± 1.7 | 7.5 ± 1.6 | 8.5 ± 1.7 | 15.6 ± 1.0* | 6.7 ± 0.4 |
| AP-5 0.3 (8) | 23.9 ± 1.5 | 13.4 ± 1.3 | 9.6 ± 1.0 | 8.6 ± 1.3 | 10.3 ± 1.1 | 16.5 ± 1.5* | 9.9 ± 0.7 | |
| DNQX 0.005 (9) | 23.3 ± 1.4 | 13.9 ± 0.9 | 7.9 ± 1.1 | 7.9 ± 1.1 | 7.7 ± 1.3 | 14.4 ± 2.2* | 5.9 ± 0.9 | |
| 4 | Control (8) | 21.4 ± 1.4 | 13.2 ± 1.9 | 7.8 ± 1.4 | 7.4 ± 1.7 | 7.4 ± 1.5 | 13.6 ± 1.4* | 6.1 ± 0.6 |
| AP-5 0.15 (8) | 24.7 ± 2.1 | 14.8 ± 2.1 | 8.3 ± 0.9 | 7.8 ± 1.1 | 8.5 ± 0.8 | 13.4 ± 1.4* | 6.9 ± 0.8 | |
| DNQX 0.001 (7) | 24.6 ± 1.7 | 16.4 ± 1.4 | 7.5 ± 0.9 | 7.2 ± 0.9 | 7.8 ± 1.1 | 3.4 ± 0.91-160 | 2.8 ± 0.81-160 | |
| 5 | Control (7) | 15.9 ± 0.8 | 8.7 ± 1.0 | 6.9 ± 1.2 | 6.6 ± 1.2 | 7.1 ± 1.1 | 15.1 ± 1.9* | 5.8 ± 1.0 |
| DNQX 0.001 (7) | 10.6 ± 2.3 | 4.2 ± 2.2 | 1.9 ± 0.5 | 1.1 ± 0.6 | 1.3 ± 0.5 | 8.8 ± 1.0† | 8.7 ± 1.01-160 | |
The duration of contact is expressed as mean per object. Mean duration of contact with the entire set of objects before the spatial change, S2–S4, and with the different object categories, DO and NDO, before (S4) and after (S5) the change.
p < 0.05 within DO group versus NDO group in S5;
F1-160: †p < 0.05 within object category saline versus drug-treated mice in S5.
Experiment 1: effect of post-training AP-5 administration into the nucleus accumbens on detection of spatial change
Table 1 shows the mean time spent in contact with the different object categories in S5 by saline- and drug-injected animals. Mice injected with saline immediately after training explored DO more than NDO. The difference in the time spent exploring the two categories of objects was reduced in a dose-dependent manner by focal administrations of AP-5 after training. Mice injected with the lower dose explored DO significantly more than NDO, whereas animals treated with the two higher doses showed no significant difference between the two object categories.
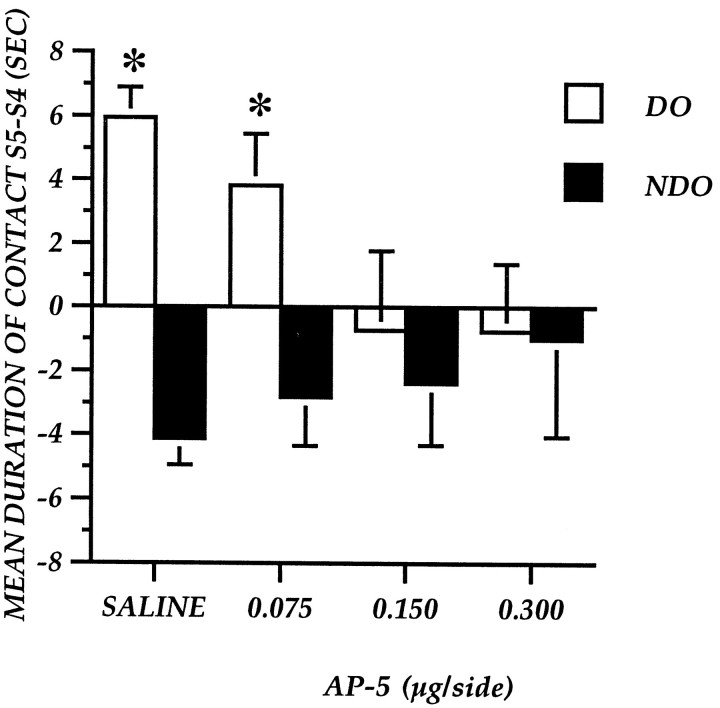
Figure 3 represents the effect of post-trial AP-5 administrations on the exploration of the two categories of objects, expressed as difference between S5 and S4. AP-5 injections decrease the re-exploration of DO without affecting NDO. The one-between one-within analysis revealed a significant effect of the object category (F(1,33) = 39.887;p = 0.0001) and a significant interaction between drug and object category (F(1,33) = 10.731;p = 0.0001) but no significant drug effect (F(3,33) = 0.537; p = 0.66).
Fig. 3.
Experiment 1: Reactivity to spatial change of mice after immediate post-training focal administration of AP-5 into the nucleus accumbens. The histogram illustrates the mean time (± SEM) spent exploring the DO or NDO in S5 minus the time spent exploring the same class of objects in the last session of habituation (S4). *p < 0.05; DO versus NDO.
Experiment 2: effect of post-training DNQX administration into the nucleus accumbens on detection of spatial change
Table 1 shows absolute values for DO and NDO exploration in S5. S5 control animals as well as DNQX-injected mice spent more time in contact with DO compared with NDO. Post hoc analysis revealed a significant difference between the two categories in all groups.
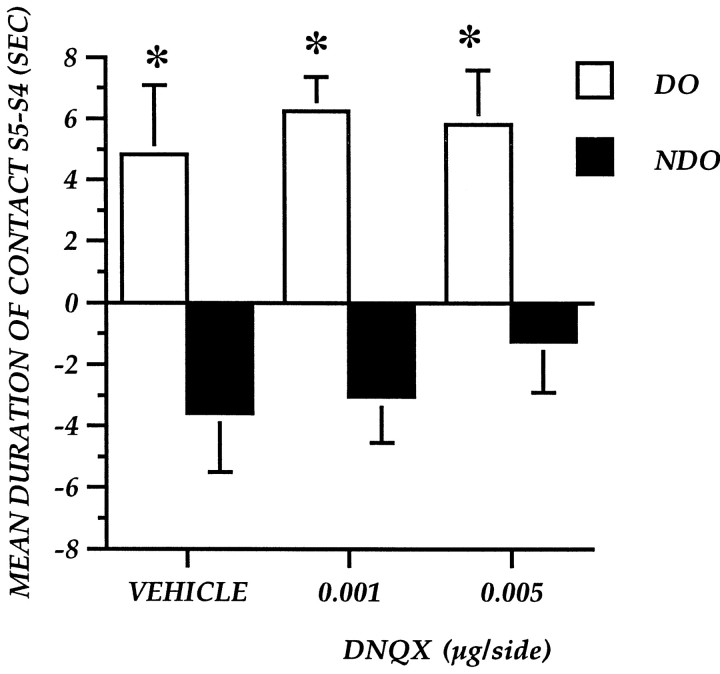
Figure 4 represents the effect of immediate post-training DNQX administrations on DO–NDO exploration, expressed as the difference between S5 and S4. The one-between one-within factor analysis revealed a significant main object category effect (F(1,19) = 40.6;p = 0.0001) but no treatment effect (F(2,19) = 0.397; p = 0.6778) and no treatment–object category interaction (F(2,19) = 0.263; p = 0.7718).
Fig. 4.
Experiment 2: Reactivity to spatial change of mice after immediate post-training focal administration of the AMPA antagonist DNQX into the nucleus accumbens. The histogram illustrates the mean time (± SEM) spent exploring the DO or NDO in S5 minus the time spent exploring the same class of objects in the last session of habituation (S4). *p < 0.05; DO versus NDO.
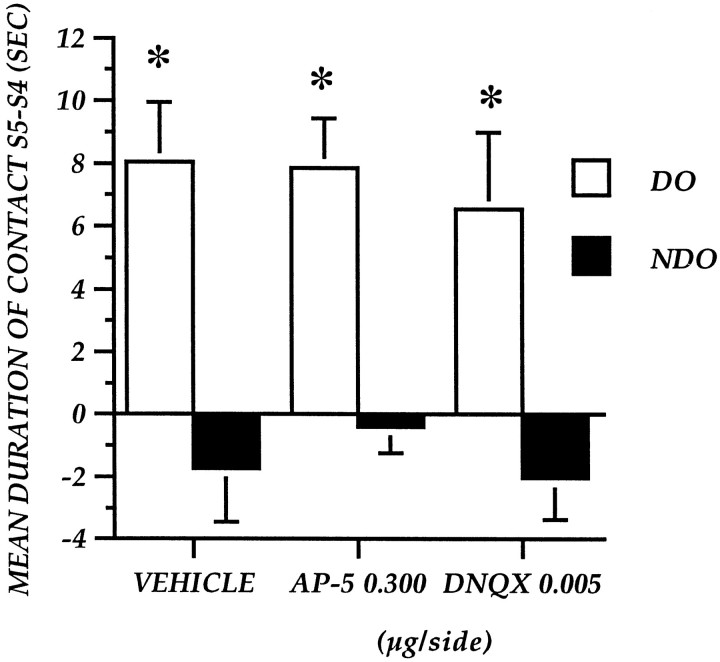
Experiment 3: effect of AP-5 and DNQX administration into the nucleus accumbens at 120 min after training on detection of spatial change
The absolute values for DO–NDO exploration in S5 after administrations of vehicle, NMDA antagonist, and AMPA antagonist 120 min after training are reported in Table 1. Control mice showed a higher level of DO exploration rather than NDO exploration. Focal administrations of AP-5 and DNQX did not impair this response.
Figure 5 represents the effects of AP-5 and DNQX injections administered 120 min after training on DO and NDO exploration, expressed as difference in two subsequent sessions (S5–S4). The ANOVA revealed a mean object category effect (F(1,23) = 91.087; p = 0.0001) but no significant treatment effect (F(2,23) = 0.231; p = 0.796) or interaction between the two factors (F(2,23) = 0.244; p = 0.785).
Fig. 5.
Experiment 3: Reactivity to spatial change of mice after post-training focal administration of AP-5 at 0.03 μg/side and DNQX at 0.005 μg/side into the nucleus accumbens 120 min after S4. The histogram illustrates the mean time (± SEM) spent exploring the DO or NDO in S5 minus the time spent exploring the same class of objects in the last session of habituation (S4). *p < 0.05; DO versus NDO.
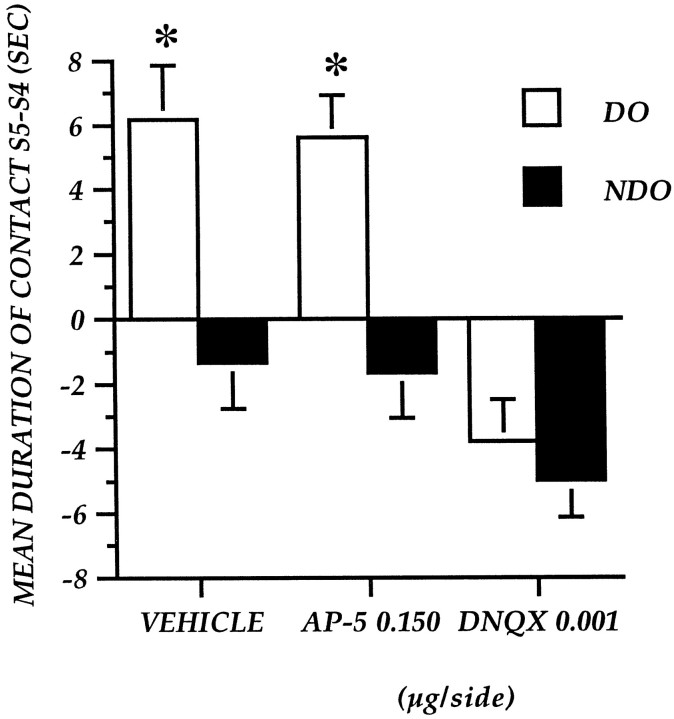
Experiment 4: effect of pretest AP-5 and DNQX administration into the nucleus accumbens on detection of spatial change
Table 1 shows the mean time of contact with DO and NDO in S5. Control mice explored DO more than NDO. Pretest administrations of the NMDA antagonist did not affect the amount of time spent exploring DO and NDO in S5 as well as the difference between the two object categories. On the contrary, pretest DNQX injections decreased exploration of both DO and NDO as well as the difference between DO and NDO.
Figure 6 represents the effects of focal administrations of vehicle and drugs immediately before the test, expressed as a difference in the exploration of the two object categories in the two subsequent sessions. The ANOVA revealed a significant object category effect (F(1,20) = 60.458; p = 0.0001), a mean drug effect (F(2,20) = 8.519; p = 0.002), and a significant interaction between the two factors (F(2,20) = 8.653; p = 0.002).
Fig. 6.
Experiment 4: Reactivity to spatial change of mice after pretest focal administration of AP-5 at 0.015 μg/side and DNQX at 0.001 μg/side into the nucleus accumbens. The histogram illustrates the mean time (± SEM) spent exploring the DO or NDO in S5 minus the time spent exploring the same class of objects in the last session of habituation (S4). *p < 0.05; DO versus NDO.
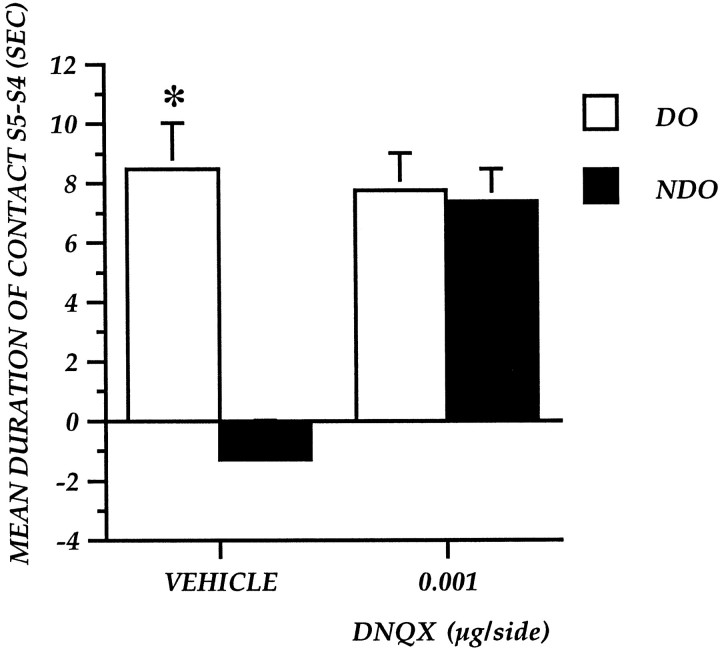
Experiment 5: effect of pretraining DNQX administration into the nucleus accumbens on detection of spatial change
Table 1 shows the mean time of contact with DO and NDO in S5. Pretraining DNQX administration induced a decrease in the time spent exploring DO but increased the time spent exploring NDO, thus reducing the difference in object category exploration observed in control mice.
Figure 7 shows the effects of pretraining vehicle and DNQX administrations expressed as a difference between S5 and S4 for the two object categories. The ANOVA revealed a main drug effect (F(1,12) = 5.2;p = 0.04), a main object effect (F(1,12) = 66.36; p = 0.0001), and an interaction between the two main effects (F(1,12) = 57.1; p = 0.001).
Fig. 7.
Experiment 5: Reactivity to spatial change of mice after pretraining focal administration of DNQX at 0.001 μg/side into the nucleus accumbens. The histogram illustrates the mean time (± SEM) spent exploring the DO or NDO in S5 minus the time spent exploring the same class of objects in the last session of habituation (S4). *p < 0.05; DO versus NDO.
DISCUSSION
The results presented in this paper confirm and extend earlier studies on the involvement of glutamate receptors located within the Nacc in processing spatial information (Maldonado-Irizarry and Kelley, 1995; Floresco et al., 1997; Usiello et al., 1998; Sargolini et al., 1999; Smith-Roe et al., 1999). In this study, we demonstrate that (1) immediate post-training focal administrations into the Nacc of the NMDA antagonist AP-5 impair detection of a spatial change on the test day (24 hr later); (2) immediate pretest AP-5 infusions or AP-5 infusions administered 120 min after the training do not affect detection of a spatial change; (3) immediate focal administrations of the AMPA antagonist DNQX into the Nacc or administrations 120 min after training do not affect detection of spatial change on the test day; and (4) pretest and pretraining administrations of DNQX significantly impair spatial change detection.
There is considerable evidence suggesting an involvement of the Nacc in spatial learning (Annett et al., 1989; Seamans and Phillips, 1994;Maldonado-Irizarry and Kelley, 1995; Floresco et al., 1996, 1997;Roullet et al., 1997; Usiello et al., 1998). However, very few studies have investigated the role of this structure in memory consolidation (Lorenzini et al., 1995; Setlow and McGaugh, 1998). Post-trial manipulations have the advantage of avoiding possible effects on learning and are considered to act on the consolidation process (McGaugh, 1966; Gold and McGaugh, 1975, 1989). In the present study, AP-5 administered immediately after training produced an impairment of spatial detection when mice were tested for retention 24 hr later. It is therefore unlikely that AP-5 was still effective on the test day. Furthermore, administrations of the highest dose of the NMDA antagonist 120 min after testing did not affect reactivity to spatial change on the test day, suggesting a specific effect of NMDA receptor blockade in this structure on the consolidation of spatial information rather than an aspecific effect of the drug on retention test performance. Finally, AP-5 administrations before testing did not affect performance. These data demonstrate that NMDA receptors located in the Nacc are specifically necessary for memory consolidation. On the contrary, the retrieval of spatial information does not need the activation of NMDA receptors located in this structure. This evidence is consistent with the lack of effect observed after NMDA receptor blockade in the same structure with regard to retrieval in a nonspatial task (Kelley et al., 1997).
Previous studies have indicated an involvement of Nacc in memory consolidation on the basis of the impairing effects induced by post-trial temporary lesions with TTX or blockade of D2 dopamine receptors, respectively, in passive avoidance tasks, and in the spatial version of the Morris water maze (Lorenzini et al., 1995; Setlow and McGaugh, 1998). The present study confirms and expands these previous observations, demonstrating an involvement of the NMDA receptors located into the Nacc in the consolidation of spatial information in a nonassociative task.
The purpose of this study was also to verify possible different roles of the two major classes of glutamate receptors, AMPA and NMDA, in the distinct stages of memory formation. We have recently demonstrated an involvement of both glutamate receptors classes located in this structure in the acquisition of spatial information (Usiello et al., 1998; Sargolini et al., 1999). Similar conclusions have been drawn on the basis of an appetitively motivated spatial task (Maldonado-Irizarry and Kelley, 1995; Smith-Roe et al., 1999). In the present study, we did not find any effect of immediate post-training administrations of the AMPA antagonist DNQX on reactivity to spatial change 24 hr later. In addition, we did not find any effect of DNQX when administered 120 min after training. These data demonstrate that this glutamate receptor class is not implicated in memory consolidation as assessed in this spatial task. To our knowledge, there are no reports in the literature on the possible involvement of AMPA receptors in memory consolidation. It should be noted, however, that an increase in AMPA receptor binding has been found 3 hr after learning (Izquierdo and Medina, 1997). Therefore, we cannot exclude the possibility that AMPA receptor activation might be necessary in later stages of the consolidation process. Pretest and pretraining AMPA receptor blockade, however, impaired reactivity to spatial change in S5. Decreased interest of mice toward the objects during training or testing in the two experiments cannot be excluded. It should be noted, however, that when administration occurred before training, the animals were impaired in detecting the change 24 hr after drug administration. Thus it is difficult to ascribe this effect simply to an action of the drug on the test phase. To detect the change, the mice have to correctly perceive the environment during training to constitute a map and correctly perceive this environment in the test phase to detect a mismatch between the stored map and the actual disposition of the objects. It is of interest that DNQX impairs both phases, supporting the suggestion that AMPA receptors might be needed to process information to guide ongoing behavior (Maldonado-Irizarry and Kelley, 1995).
It has been demonstrated in the rat that the core rather than the shell subregion of the accumbens is involved in processing of spatial information (Maldonado-Irizarry and Kelley, 1995). Core–shell differences were not investigated in the present study, and even if a small volume was injected, drug diffusion in both regions cannot be completely excluded. However, it is interesting to note that most injection sites were located in the core of the Nacc, thus suggesting that core might also play a relevant role in spatial learning in mice accumbens.
The task we used in this study, modified from Poucet (1989), is designed to estimate the ability of rodents to encode spatial relationships among discrete stimuli (Poucet, 1989; Thinus-Blanc et al., 1992; Roullet et al., 1996, Usiello et al., 1998). Our interest in this behavioral paradigm pertains to the fact that, in contrast to other learning tasks, mice do not have to form any kind of association between stimulus and response, and no explicit (positive or negative) reinforcement is present in this procedure. The role of Nacc in mediating reward-related learning has been well assessed (Robbins and Everitt, 1996; Kelley et al., 1997); therefore, one might hypothesize that the Nacc is involved in processing information related to rewarding stimuli. The data presented in this paper suggest that the Nacc plays a pivotal role not only in the acquisition (Usiello et al., 1998; Sargolini et al., 1999) but also in the consolidation of spatial information necessary to guide behavior even when no explicit reinforcement is present or stimulus–response association is necessary. This implies a more general role played by the Nacc in processing contextual information.
The Nacc is viewed as an interface between the limbic and the motor systems that acts to select the most appropriate behavioral response (Mogenson, 1987; Mizumori et al., 1999). In this framework, limbic afferent from the hippocampus, amygdala, and prefrontal cortex would relay contextual information that is integrated within this structure to guide ongoing behavior on the basis of current context. Previous learning helps spatial navigation; therefore, it is conceivable that an integration between stored and perceived information might occur within the Nacc. Moreover, an involvement of this structure in further stages of information processes is also possible. Information flow from these structures depends on intact glutamatergic transmission that acts on AMPA and NMDA receptors in the Nacc. In this paper, we dissociate the role of the two glutamate receptor classes, demonstrating that NMDA receptors are involved in consolidation but not ongoing discrimination, whereas AMPA receptors seem to have an opposite role.
Even if experimental evidence on the involvement of the Nacc in consolidation of spatial information is now accumulating, whether this structure plays a role in information storage or whether memory resides elsewhere is still unclear. Several studies have demonstrated plastic changes involving glutamatergic afference to the Nacc (Mulder et al., 1997), and it has been suggested that a reorganization of dendritic spines within this structure might be the basis for adaptive or maladaptive changes after repeated psychostimulant administrations (Robinson and Kolb, 1997). This evidence suggests that spatial information might at least be partially stored within the Nacc.
Finally, it should be noted that deafferentation studies and pharmacological manipulation of the Nacc (Floresco et al., 1996;Sargolini et al., 1999; Setlow et al., 2000) demonstrate that each of the different limbic structures plays a selective role in processing information. However, the exact neurobiological substrate of the interaction among the different projections remains to be determined.
Footnotes
This study was supported in part by the 40% Ministero dell'Universitá e della Ricerca Scientifica e Tecnologica grants “Farmacologia dell'apprendimento e della memoria” and “Neurobiologia delle tossicodipendenze e dei meccanismi di gratificazione naturale” and by a grant from the University of Rome “La Sapienza” (A.O.).
Correspondence should be addressed to Andrea Mele, Dipartimento di Genetica e Biologia Molecolare, Università di Roma “La Sapienza,” Ple. Aldo Moro, 5, I-00185, Rome, Italy. E-mail:Andrea.Mele@uniroma1.it.
REFERENCES
- 1.Albin RL, Makowiec RL, Hollingsworth Z, Dure IVLS, Penney JB, Young AB. Excitatory amino acid binding sites in the basal ganglia of the rat: a quantitative autoradiographic study. Neuroscience. 1991;46:35–48. doi: 10.1016/0306-4522(92)90006-n. [DOI] [PubMed] [Google Scholar]
- 2.Annett LE, McGregor A, Robbins TW. The effects of ibotenic acid lesions of the nucleus accumbens on spatial learning and extinction in the rat. Behav Brain Res. 1989;31:231–242. doi: 10.1016/0166-4328(89)90005-3. [DOI] [PubMed] [Google Scholar]
- 3.Beckstead RM. An autoradiographic examination of corticocortical and subcortical projections of the mediodorsal-projections (prefrontal) cortex in the rat. J Comp Neurol. 1979;184:42–62. doi: 10.1002/cne.901840104. [DOI] [PubMed] [Google Scholar]
- 4.Floresco SB, Seamans JK, Phillips AG. Differential effects of lidocaine infusions into the ventral CA1/subiculum or the nucleus accumbens on acquisition and retention of spatial information. Behav Brain Res. 1996;81:163–171. doi: 10.1016/s0166-4328(96)00058-7. [DOI] [PubMed] [Google Scholar]
- 5.Floresco SB, Seamans JK, Phillips AG. Selective roles for hippocampal, prefrontal cortical, and ventral striatal circuits in radial-arm maze tasks with or without a delay. J Neurosci. 1997;17:1880–1890. doi: 10.1523/JNEUROSCI.17-05-01880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin BJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic; San Diego: 1997. [Google Scholar]
- 7.Gold PE, McGaugh JL. A single trace, two process view of memory processes. In: Deutsch D, Deutsch JA, editors. Short-term memory. Academic; New York: 1975. pp. 355–378. [Google Scholar]
- 8.Graybiel AG. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5:733–741. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- 9.Groenewegen HJ, Vermeulen-van Der Zee E, Te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat: a study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 10.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 11.Kelley AE, Domesick VB. The distribution of the projection from the hippocampal formation to the nucleus accumbens in the rat: an anterograde- and retrograde-horseradish peroxydase study. Neuroscience. 1982;7:2321–2335. doi: 10.1016/0306-4522(82)90198-1. [DOI] [PubMed] [Google Scholar]
- 12.Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-d-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorenzini CA, Baldi E, Bucarelli C, Tassoni G. Time dependent deficits of rat's memory consolidation induced by tetrodotoxin injections into the caudate-putamen, nucleus accumbens, and globus pallidus. Neurobiol Learn Mem. 1995;63:87–93. doi: 10.1006/nlme.1995.1008. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado-Irizarry CS, Kelley AE. Excitatory amino acid receptors within nucleus accumbens subregions differentially mediate spatial learning in rat. Behav Pharmacol. 1995;6:527–539. [PubMed] [Google Scholar]
- 15.McGaugh JL. Time-dependent processes in memory storage. Science. 1966;153:1351–1358. doi: 10.1126/science.153.3742.1351. [DOI] [PubMed] [Google Scholar]
- 16.McGaugh JL. Dissociating learning and performance: drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 17.Mizumori SJY, Pratt WE, Ragozzino KE. Function of the nucleus accumbens within the context of the larger striatal system. Psychobiology. 1999;27:214–224. [Google Scholar]
- 18.Mogenson GJ. Limbic-motor integration. Prog Psychobiol Physiol Psychol. 1987;12:117–170. [Google Scholar]
- 19.Mulder AB, Arts MPM, Lopes da Silva FH. Short- and long-term plasticity of the hippocampus to nucleus accumbens and prefrontal cortex pathways in the rat, in vivo. Eur J Neurosci. 1997;9:1603–1611. doi: 10.1111/j.1460-9568.1997.tb01518.x. [DOI] [PubMed] [Google Scholar]
- 20.Poucet B. Object exploration, habituation, and response to spatial change in rats following septal or medial frontal cortical damage. Behav Neurosci. 1989;103:1009–1016. doi: 10.1037//0735-7044.103.5.1009. [DOI] [PubMed] [Google Scholar]
- 21.Robbins TW, Everitt BJ. Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 22.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roullet P, Mele A, Ammassari-Teule M. Involvement of glutamatergic and dopaminergic systems in the reactivity of mice to spatial and non-spatial change. Psychopharmacology. 1996;126:55–61. doi: 10.1007/BF02246411. [DOI] [PubMed] [Google Scholar]
- 24.Roullet P, Mele A, Ammassari-Teule M. Ibotenic acid lesions of the nucleus accumbens improve detection of spatial novelty in poor spatial learner mice. Behav Neurosci. 1997;111:976–984. doi: 10.1037//0735-7044.111.5.976. [DOI] [PubMed] [Google Scholar]
- 25.Sargolini F, Roullet P, Oliverio A, Mele A. Effects of lesions to the glutamatergic afferents to the nucleus accumbens in the modulation of reactivity to spatial and non-spatial novelty in mice. Neuroscience. 1999;93:855–867. doi: 10.1016/s0306-4522(99)00259-6. [DOI] [PubMed] [Google Scholar]
- 26.Save E, Poucet B, Foreman N, Buhot MC. Object exploration and reactions to spatial change and nonspatial changes in hooded rats following damage to parietal cortex or dorsal hippocampus. Behav Neurosci. 1992;106:447–456. [PubMed] [Google Scholar]
- 27.Schacter GB, Yang CR, Innis NK, Mogenson GJ. The role of the hippocampus-nucleus accumbens pathway in radial-arm maze performance. Brain Res. 1989;494:339–349. doi: 10.1016/0006-8993(89)90602-1. [DOI] [PubMed] [Google Scholar]
- 28.Seamans JK, Phillips AG. Selective memory impairments produced by transient lidocaine-induced lesions of the nucleus accumbens in rats. Behav Neurosci. 1994;108:456–468. doi: 10.1037//0735-7044.108.3.456. [DOI] [PubMed] [Google Scholar]
- 29.Setlow B, McGaugh JL. Sulpiride infused into the nucleus accumbens posttraining impairs memory of spatial water maze training. Behav Neurosci. 1998;112:603–610. doi: 10.1037//0735-7044.112.3.603. [DOI] [PubMed] [Google Scholar]
- 30.Setlow B, Roozendaal B, McGaugh JL. Involvement of a basolateral amygdala complex-nucleus accumbens pathway in glucocorticoid-induced modulation of memory consolidation. Eur J Neurosci. 2000;12:367–375. doi: 10.1046/j.1460-9568.2000.00911.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith-Roe SL, Sedaghian K, Kelley AE. Spatial learning and performance in the radial arm maze is impaired after N-methyl-d-aspartate (NMDA) receptor blockade in striatal subregions. Behav Neurosci. 1999;113:703–717. doi: 10.1037//0735-7044.113.4.703. [DOI] [PubMed] [Google Scholar]
- 32.Thinus-Blanc C, Durup M, Poucet B. The spatial parameter encoded by hamsters during exploration. Behav Processes. 1992;26:43–57. doi: 10.1016/0376-6357(92)90031-8. [DOI] [PubMed] [Google Scholar]
- 33.Usiello A, Sargolini F, Roullet P, Ammassari-Teule M, Passino E, Oliverio A, Mele A. N-methyl-d-aspartate receptors in the nucleus accumbens are involved in detection of spatial novelty in mice. Psychopharmacology. 1998;137:175–183. doi: 10.1007/s002130050607. [DOI] [PubMed] [Google Scholar]
- 34.Walaas I, Fonnum F. The effects of surgical and chemical on neurotransmitter candidates on the nucleus accumbens. Neuroscience. 1979;4:209–216. doi: 10.1016/0306-4522(79)90083-6. [DOI] [PubMed] [Google Scholar]
- 35.White NM. Mnemonic functions of the basal ganglia. Curr Opin Neurobiol. 1997;7:164–169. doi: 10.1016/s0959-4388(97)80004-9. [DOI] [PubMed] [Google Scholar]