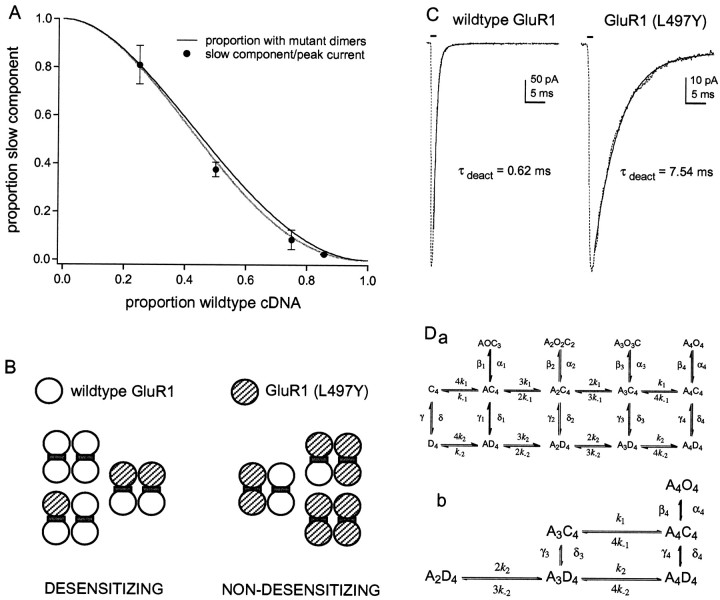
Fig. 6.
The results are consistent with the conclusion that AMPA receptors are dimers of dimers. A, The relative amplitude of the slow component is plotted as a function of the proportion of wild-type cDNA included in the transfection mixture. Each point is the mean value from measurements made in five to seven patches. The bars indicate the SD. The black curve shows the expected proportion of tetramers that contain at least one dimer with two mutant subunits. The gray curve shows the corresponding prediction after correcting for missing channels that never open using the simulated data in Figure5B. B, Diagram illustrating the partitioning of tetrameric assemblies assumed to display wild-type and mutant desensitization for the combinatorial analysis shown inA. C, Deactivation kinetics of wild-type GluR1flip (left) and homomeric GluR1flip (L497Y) channels (right). The currents were evoked by 1 msec applications (bars) of 5 mm glutamate. The decays of the currents (dotted traces) were fitted with single exponential functions (solid curves) with the indicated time constants.Da, Possible kinetic scheme illustrating some general features of AMPA receptor activation and desensitization suggested by previous work. It is assumed that the channel is a tetramer where each subunit can bind a single agonist molecule (A) and can exist in three distinct conformations: closed (C), open (O), and desensitized (D). The states in the top row are open, those in the middle row are closed, and those in the bottom row are desensitized. For closed and desensitized states, each subunit is constrained to adopt the same conformation. The affinity of agonist binding is higher affinity to desensitized states than to closed states (k−2/k2 <k−1/k1). The top two rows represent one of the models tested bySmith et al. (2000) that incorporates evidence for concentration-dependent substate gating and the presence of as many as four discrete open levels for some native AMPA receptors. The notation of the open states implies that individual subunits gate independently. For simplicity, it is assumed that binding is not cooperative, that binding and unbinding do not occur when the channel is open, that transitions between nonadjacent states do not occur, and that the probability of opening from state C4 is zero.Db, Enlarged view of the scheme showing the portion of the model most relevant to our results.