Fig. 7.
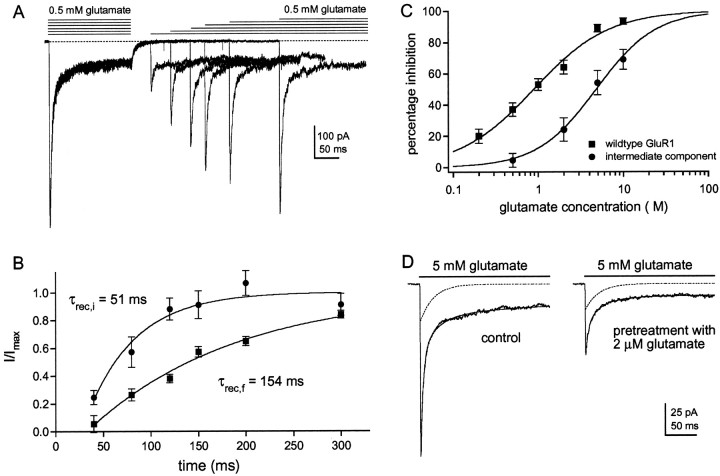
The intermediate component recovers faster from desensitization and shows reduced apparent affinity for glutamate.A, Six consecutive sweeps obtained in a patch from a cell that was cotransfected with GluR1flip (L497Y) and GluR1flip cDNAs at a ratio of 1:3. Glutamate (0.5 mm) was applied for 100 msec, and then a second application was made at a varying interval (40, 80, 120, 150, 200, 300 msec). The decays of the currents evoked by the second application of each pair were fitted with three exponential components, and their amplitudes were expressed as a proportion of the corresponding component in the decay of the current evoked by the preceding conditioning pulse.B, Graph showing the recovery from desensitization of the fast (▪) and the intermediate (●) components. Eachpoint is the mean from five different patches (bars indicate SD). Each set of results was fitted with a single exponential function. The fits did not extrapolate to zero time. This apparent offset was estimated from the fit and was subtracted from all recovery times. C, Percentage inhibition of peak currents through wild-type GluR1flip channels (▪) are plotted as a function of glutamate concentration and compared with the corresponding results for the intermediate component detected in coexpression experiments (●). Each point is the mean value obtained from five patches (bars indicate SD). Each data set was fitted with a Hill-type equation with the maximal inhibition constrained to 100%. In the coexpression experiments, glutamate concentrations above 10 μm produced significant channel activation. The fits gave IC50 values of 0.89 μm for the wild-type channels and 4.30 μm for the channels underlying the intermediate component of decay. D, Currents in a patch from a cell transfected with GluR1flip (L497Y) and GluR1flip at a cDNA ratio of 1:3. The records are responses to 5 mm glutamate before and during preincubation with 2 μm glutamate. The three-exponential fits to the decays (smooth solid lines) are superposed on the currents, and the dotted lines show the intermediate component obtained from each fit. Note that preincubation with glutamate reduces the amplitude of the fast component more than it does the amplitude of the intermediate component (71 and 30% inhibition, respectively).