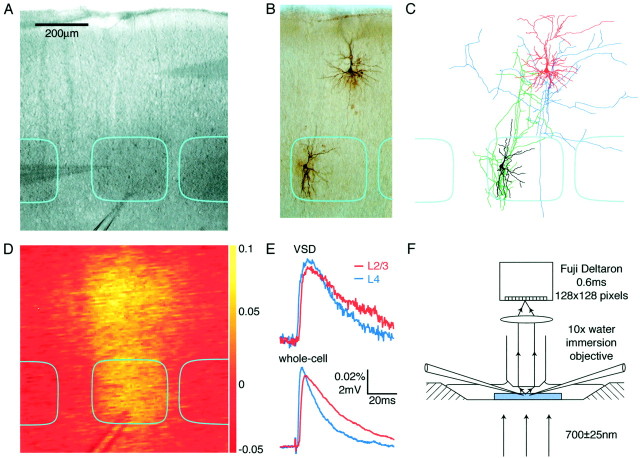
Fig. 1.
Experimental setup for simultaneous whole-cell and voltage-sensitive dye recording. A, Bright-field image of somatosensory barrel cortex from a slice stained with RH155.Dark regions correspond to the layer 4 barrels, which are outlined in cyan. Two whole-cell recording pipettes are visible, one in layer 2/3 and the other in layer 4. A large-diameter patch pipette filled with extracellular solution is used for field stimulation, with the tip positioned at the bottom of the central barrel. B, The excitatory neurons are filled with biocytin during the whole-cell recordings, allowing their structure to be visualized after fixation and staining.C, Reconstruction of the biocytin-filled layer 2/3 pyramidal neuron (reddendrite andblueaxon) and the layer 4 spiny stellate (blackdendrite and greenaxon). D, A single unfiltered differential image normalized to the bright-field transmitted light (ΔI/I0) of the voltage-sensitive dye signal taken 12 msec after stimulation.E, Time course of voltage-sensitive dye responses (top traces) compared with the simultaneously obtained whole-cell recordings (bottom traces). The voltage-sensitive dye signals were quantified from an area of 50 × 50 μm around the location of the neurons from which the whole-cell recordings are made (layer 2/3 red traces and layer 4blue traces). F, Optical arrangement for simultaneous whole-cell recordings and voltage-sensitive dye recordings. The slice is illuminated with 700 nm light from a halogen lamp and viewed with a 10× water immersion lens. The slice is stained with voltage-sensitive dye RH155, which increases its absorption of 700 nm light when membranes are depolarized. Small changes in the amount of light transmitted through the slice can be recorded with a Fuji Deltaron camera. With a square field of view of 775 μm obtained under these conditions, each pixel covers a region of 6 × 6 μm, and each frame has 0.6 msec duration.