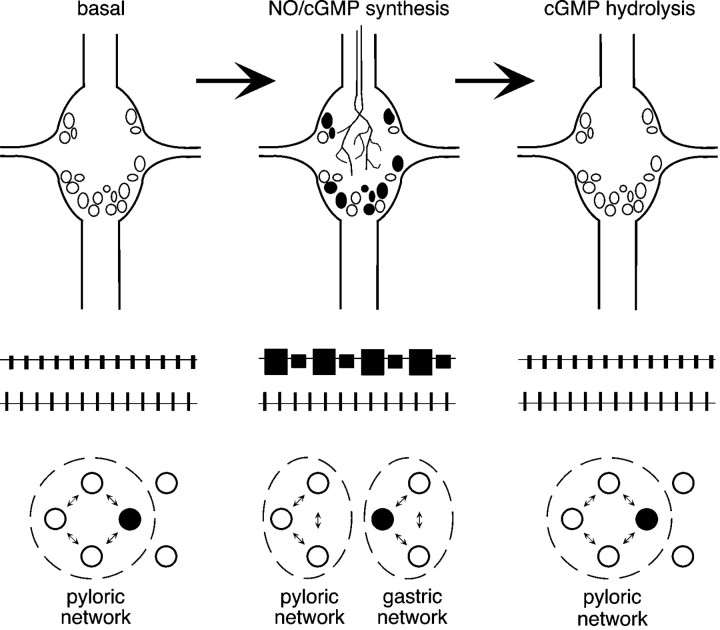
Fig. 9.
A proposed role for NO–cGMP neuromodulation in the rapid and reversible partitioning of STG motor networks. This model assumes that NO–cGMP signaling interacts with parallel modulatory inputs (data not shown), which are intact and active.Left, Between bouts of feeding, NO–cGMP neuromodulation is absent. The pyloric network is active, and gastric mill units are either silent (open circles) or participate in the pyloric rhythm (filled circle).Middle, When food reaches the gastric mill, NO is released into the STG neuropil from descending inputs or another as yet unidentified source. cGMP accumulates in a subset of neurons, and motor output from the ganglion is partitioned into two parallel rhythms. Previously silent gastric mill neurons become active, whereas others pattern switch from the pyloric network to the gastric mill network (filled circle). Right, NO release stops when food is no longer present in the foregut. cGMP is rapidly hydrolyzed, and the STG returns to a unitary network configuration.