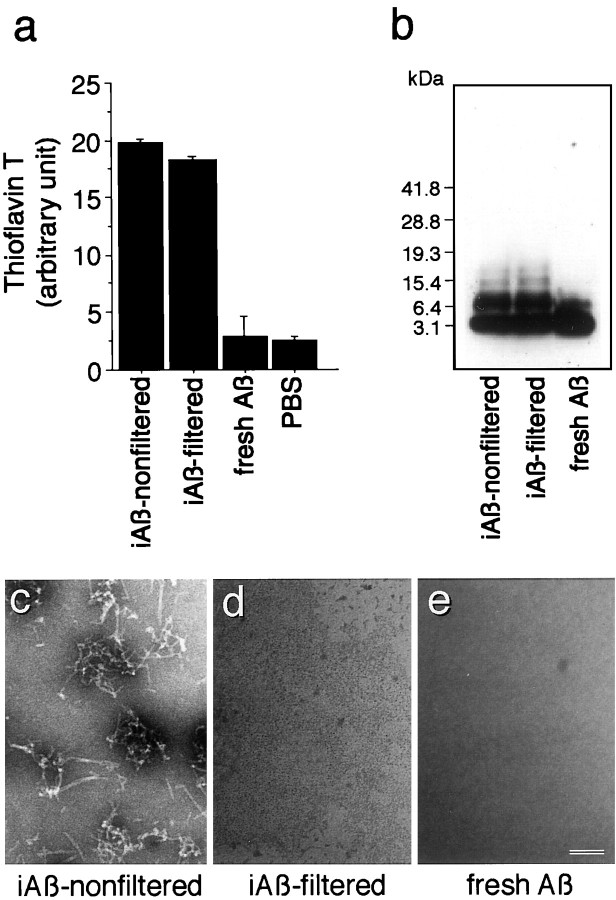
Fig. 1.
Characterization of Aβ1–40. Aβ1–40 was prepared as described in Materials and Methods. a, The aliquots of iAβ-nonfiltered,iAβ-filtered, fresh Aβ, and PBS were subjected to thioflavin-T assays as described in Materials and Methods. Three independent experiments were performed, and similar results were obtained.b, The equal volume of 2× sample solubilizing buffer was added to each Aβ solution, of which the concentration was normalized with PBS. The samples were then subjected to 4–20% Tris/tricine SDS-PAGE, followed by Western blot analysis.c, Electron micrograph of each sample is shown. The samples were centrifuged at 34,500 rpm for 48 hr using a SW 41-Ti rotor. Electron microscopic analysis of the lower part of each solution containing the resuspended pellet was performed. Results of negative staining show that fibrillar structures are found in the sample ofiAβ-nonfiltered(c); however, no fibril is detected in the samples of iAβ-filtered(d) or fresh Aβ (e). Scale bar, 50 nm.